An Abbreviated Protocol for In Vitro Generation of Functional Human Embryonic Stem Cell-Derived Beta-Like Cells
- PMID: 27755557
- PMCID: PMC5068782
- DOI: 10.1371/journal.pone.0164457
An Abbreviated Protocol for In Vitro Generation of Functional Human Embryonic Stem Cell-Derived Beta-Like Cells
Abstract
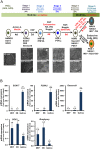
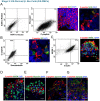
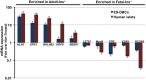
The ability to yield glucose-responsive pancreatic beta-cells from human pluripotent stem cells in vitro will facilitate the development of the cell replacement therapies for the treatment of Type 1 Diabetes. Here, through the sequential in vitro targeting of selected signaling pathways, we have developed an abbreviated five-stage protocol (25-30 days) to generate human Embryonic Stem Cell-Derived Beta-like Cells (ES-DBCs). We showed that Geltrex, as an extracellular matrix, could support the generation of ES-DBCs more efficiently than that of the previously described culture systems. The activation of FGF and Retinoic Acid along with the inhibition of BMP, SHH and TGF-beta led to the generation of 75% NKX6.1+/NGN3+ Endocrine Progenitors. The inhibition of Notch and tyrosine kinase receptor AXL, and the treatment with Exendin-4 and T3 in the final stage resulted in 35% mono-hormonal insulin positive cells, 1% insulin and glucagon positive cells and 30% insulin and NKX6.1 co-expressing cells. Functionally, ES-DBCs were responsive to high glucose in static incubation and perifusion studies, and could secrete insulin in response to successive glucose stimulations. Mitochondrial metabolic flux analyses using Seahorse demonstrated that the ES-DBCs could efficiently metabolize glucose and generate intracellular signals to trigger insulin secretion. In conclusion, targeting selected signaling pathways for 25-30 days was sufficient to generate ES-DBCs in vitro. The ability of ES-DBCs to secrete insulin in response to glucose renders them a promising model for the in vitro screening of drugs, small molecules or genes that may have potential to influence beta-cell function.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures





Similar articles
-
Human embryonic stem cell differentiation into insulin secreting β-cells for diabetes.Cell Biol Int. 2012 Nov 1;36(11):1013-20. doi: 10.1042/CBI20120210. Cell Biol Int. 2012. PMID: 22897387
-
PDGF Facilitates Direct Lineage Reprogramming of Hepatocytes to Functional β-Like Cells Induced by Pdx1 and Ngn3.Cell Transplant. 2016 Oct;25(10):1893-1909. doi: 10.3727/096368916X691439. Cell Transplant. 2016. PMID: 27076404
-
Anterior-Posterior Patterning of Definitive Endoderm Generated from Human Embryonic Stem Cells Depends on the Differential Signaling of Retinoic Acid, Wnt-, and BMP-Signaling.Stem Cells. 2016 Nov;34(11):2635-2647. doi: 10.1002/stem.2428. Epub 2016 Jul 4. Stem Cells. 2016. PMID: 27299363
-
Transdifferentiation of hepatic oval cells into pancreatic islet beta-cells.Front Biosci (Landmark Ed). 2012 Jun 1;17(7):2391-5. doi: 10.2741/4060. Front Biosci (Landmark Ed). 2012. PMID: 22652787 Review.
-
The new generation of beta-cells: replication, stem cell differentiation, and the role of small molecules.Rev Diabet Stud. 2010 Summer;7(2):93-104. doi: 10.1900/RDS.2010.7.93. Epub 2010 Aug 10. Rev Diabet Stud. 2010. PMID: 21060968 Free PMC article. Review.
Cited by
-
Small Molecule-Induced Pancreatic β-Like Cell Development: Mechanistic Approaches and Available Strategies.Int J Mol Sci. 2020 Mar 30;21(7):2388. doi: 10.3390/ijms21072388. Int J Mol Sci. 2020. PMID: 32235681 Free PMC article. Review.
-
Different developmental histories of beta-cells generate functional and proliferative heterogeneity during islet growth.Nat Commun. 2017 Sep 22;8(1):664. doi: 10.1038/s41467-017-00461-3. Nat Commun. 2017. PMID: 28939870 Free PMC article.
-
Signaling Molecules Regulating Pancreatic Endocrine Development from Pluripotent Stem Cell Differentiation.Int J Mol Sci. 2020 Aug 15;21(16):5867. doi: 10.3390/ijms21165867. Int J Mol Sci. 2020. PMID: 32824212 Free PMC article. Review.
-
Afadin and RhoA control pancreatic endocrine mass via lumen morphogenesis.Genes Dev. 2017 Dec 1;31(23-24):2376-2390. doi: 10.1101/gad.307637.117. Epub 2018 Jan 12. Genes Dev. 2017. PMID: 29330353 Free PMC article.
-
Ameliorating and refining islet organoids to illuminate treatment and pathogenesis of diabetes mellitus.Stem Cell Res Ther. 2024 Jun 27;15(1):188. doi: 10.1186/s13287-024-03780-7. Stem Cell Res Ther. 2024. PMID: 38937834 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

