Monocytes/Macrophages Upregulate the Hyaluronidase HYAL1 and Adapt Its Subcellular Trafficking to Promote Extracellular Residency upon Differentiation into Osteoclasts
- PMID: 27755597
- PMCID: PMC5068775
- DOI: 10.1371/journal.pone.0165004
Monocytes/Macrophages Upregulate the Hyaluronidase HYAL1 and Adapt Its Subcellular Trafficking to Promote Extracellular Residency upon Differentiation into Osteoclasts
Abstract
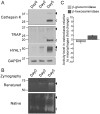
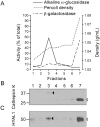
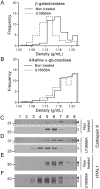
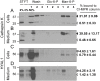
Osteoclasts are giant bone-resorbing cells originating from monocytes/macrophages. During their differentiation, they overexpress two lysosomal enzymes, cathepsin K and TRAP, which are secreted into the resorption lacuna, an acidified sealed area in contact with bone matrix where bone degradation takes place. Here we report that the acid hydrolase HYAL1, a hyaluronidase able to degrade the glycosaminoglycans hyaluronic acid (HA) and chondroitin sulfate, is also upregulated upon osteoclastogenesis. The mRNA expression and protein level of HYAL1 are markedly increased in osteoclasts differentiated from RAW264.7 mouse macrophages or primary mouse bone marrow monocytes compared to these precursor cells. As a result, the HYAL1-mediated HA hydrolysis ability of osteoclasts is strongly enhanced. Using subcellular fractionation, we demonstrate that HYAL1 proteins are sorted to the osteoclast lysosomes even though, in contrast to cathepsin K and TRAP, HYAL1 is poorly mannose 6-phosphorylated. We reported previously that macrophages secrete HYAL1 proforms by constitutive secretion, and that these are recaptured by the cell surface mannose receptor, processed in endosomes and sorted to lysosomes. Present work highlights that osteoclasts secrete HYAL1 in two ways, through lysosomal exocytosis and constitutive secretion, and that these cells promote the extracellular residency of HYAL1 through downregulation of the mannose receptor. Interestingly, the expression of the other main hyaluronidase, HYAL2, and of lysosomal exoglycosidases involved in HA degradation, does not increase similarly to HYAL1 upon osteoclastogenesis. Taken together, these findings point out the predominant involvement of HYAL1 in bone HA metabolism and perhaps bone remodeling via the resorption lacuna.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



Similar articles
-
Hyaluronidase 1 deficiency decreases bone mineral density in mice.Sci Rep. 2022 Jun 16;12(1):10142. doi: 10.1038/s41598-022-14473-7. Sci Rep. 2022. PMID: 35710820 Free PMC article.
-
Subcellular trafficking and activity of Hyal-1 and its processed forms in murine macrophages.Traffic. 2014 May;15(5):500-15. doi: 10.1111/tra.12162. Epub 2014 Mar 11. Traffic. 2014. PMID: 24502338
-
BSP and RANKL induce osteoclastogenesis and bone resorption synergistically.J Bone Miner Res. 2005 Sep;20(9):1669-79. doi: 10.1359/JBMR.050511. Epub 2005 May 16. J Bone Miner Res. 2005. PMID: 16059638
-
Genetic Deficiencies of Hyaluronan Degradation.Cells. 2024 Jul 16;13(14):1203. doi: 10.3390/cells13141203. Cells. 2024. PMID: 39056785 Free PMC article. Review.
-
[Osteoclast size regulation and its mechanism].Zhonghua Kou Qiang Yi Xue Za Zhi. 2016 Jan;51(1):58-64. doi: 10.3760/cma.j.issn.1002-0098.2016.01.014. Zhonghua Kou Qiang Yi Xue Za Zhi. 2016. PMID: 26792190 Review. Chinese.
Cited by
-
Subcellular Trafficking of Mammalian Lysosomal Proteins: An Extended View.Int J Mol Sci. 2016 Dec 28;18(1):47. doi: 10.3390/ijms18010047. Int J Mol Sci. 2016. PMID: 28036022 Free PMC article. Review.
-
Ischemic stroke disrupts the endothelial glycocalyx through activation of proHPSE via acrolein exposure.J Biol Chem. 2020 Dec 25;295(52):18614-18624. doi: 10.1074/jbc.RA120.015105. Epub 2020 Oct 30. J Biol Chem. 2020. PMID: 33127645 Free PMC article.
-
Hyaluronidase 1 deficiency decreases bone mineral density in mice.Sci Rep. 2022 Jun 16;12(1):10142. doi: 10.1038/s41598-022-14473-7. Sci Rep. 2022. PMID: 35710820 Free PMC article.
-
Epidermal keratinocytes regulate hyaluronan metabolism via extracellularly secreted hyaluronidase 1 and hyaluronan synthase 3.J Biol Chem. 2024 Jul;300(7):107449. doi: 10.1016/j.jbc.2024.107449. Epub 2024 Jun 4. J Biol Chem. 2024. PMID: 38844132 Free PMC article.
-
Hyal1 Expression in Colorectal Carcinoma Cell Migration and Invasiveness: Significance and Mechanism.Evid Based Complement Alternat Med. 2022 Jul 5;2022:4418300. doi: 10.1155/2022/4418300. eCollection 2022. Evid Based Complement Alternat Med. 2022. Retraction in: Evid Based Complement Alternat Med. 2023 Jul 19;2023:9765657. doi: 10.1155/2023/9765657. PMID: 35836827 Free PMC article. Retracted.
References
-
- Väänänen HK, Zhao H, Mulari M, Halleen JM. The cell biology of osteoclast function. J Cell Sci. 2000;113: 377–381. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

