Bmi1 Loss in the Organ of Corti Results in p16ink4a Upregulation and Reduced Cell Proliferation of Otic Progenitors In Vitro
- PMID: 27755610
- PMCID: PMC5068820
- DOI: 10.1371/journal.pone.0164579
Bmi1 Loss in the Organ of Corti Results in p16ink4a Upregulation and Reduced Cell Proliferation of Otic Progenitors In Vitro
Abstract
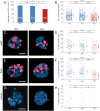
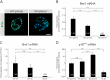
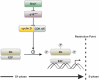
The mature mammalian organ of Corti does not regenerate spontaneously after injury, mainly due to the absence of cell proliferation and the depletion of otic progenitors with age. The polycomb gene B lymphoma Mo-MLV insertion region 1 homolog (Bmi1) promotes proliferation and cell cycle progression in several stem cell populations. The cell cycle inhibitor p16ink4a has been previously identified as a downstream target of Bmi1. In this study, we show that Bmi1 is expressed in the developing inner ear. In the organ of Corti, Bmi1 expression is temporally regulated during embryonic and postnatal development. In contrast, p16ink4a expression is not detectable during the same period. Bmi1-deficient mice were used to investigate the role of Bmi1 in cochlear development and otosphere generation. In the absence of Bmi1, the postnatal organ of Corti displayed normal morphology at least until the end of the first postnatal week, suggesting that Bmi1 is not required for the embryonic or early postnatal development of the organ of Corti. However, Bmi1 loss resulted in the reduced sphere-forming capacity of the organ of Corti, accompanied by the decreased cell proliferation of otic progenitors in otosphere cultures. This reduced proliferative capacity was associated with the upregulation of p16ink4a in vitro. Viral vector-mediated overexpression of p16ink4a in wildtype otosphere cultures significantly reduced the number of generated otospheres in vitro. The findings strongly suggest a role for Bmi1 as a promoter of cell proliferation in otic progenitor cells, potentially through the repression of p16ink4a.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




Similar articles
-
Bmi1 promotes hepatic stem cell expansion and tumorigenicity in both Ink4a/Arf-dependent and -independent manners in mice.Hepatology. 2010 Sep;52(3):1111-23. doi: 10.1002/hep.23793. Hepatology. 2010. PMID: 20648475
-
Bmi1 regulates murine intestinal stem cell proliferation and self-renewal downstream of Notch.Development. 2015 Jan 1;142(1):41-50. doi: 10.1242/dev.107714. Epub 2014 Dec 5. Development. 2015. PMID: 25480918
-
Antagonistic interplay between necdin and Bmi1 controls proliferation of neural precursor cells in the embryonic mouse neocortex.PLoS One. 2014 Jan 2;9(1):e84460. doi: 10.1371/journal.pone.0084460. eCollection 2014. PLoS One. 2014. PMID: 24392139 Free PMC article.
-
Bmi1, stem cells, and senescence regulation.J Clin Invest. 2004 Jan;113(2):175-9. doi: 10.1172/JCI20800. J Clin Invest. 2004. PMID: 14722607 Free PMC article. Review.
-
A protein synthesis brake for hematopoietic stem cell maintenance.Genes Dev. 2022 Aug 1;36(15-16):871-873. doi: 10.1101/gad.350107.122. Genes Dev. 2022. PMID: 36207141 Free PMC article. Review.
Cited by
-
The Crucial Roles of Bmi-1 in Cancer: Implications in Pathogenesis, Metastasis, Drug Resistance, and Targeted Therapies.Int J Mol Sci. 2022 Jul 26;23(15):8231. doi: 10.3390/ijms23158231. Int J Mol Sci. 2022. PMID: 35897796 Free PMC article. Review.
-
Microarray analyses of otospheres derived from the cochlea in the inner ear identify putative transcription factors that regulate the characteristics of otospheres.PLoS One. 2017 Jun 29;12(6):e0179901. doi: 10.1371/journal.pone.0179901. eCollection 2017. PLoS One. 2017. PMID: 28662075 Free PMC article.
References
-
- Roberson DW, Rubel EW. Cell division in the gerbil cochlea after acoustic trauma. Am J Otol. 1994;15(1):28–34. . - PubMed
-
- Yamasoba T, Kondo K, Miyajima C, Suzuki M. Changes in cell proliferation in rat and guinea pig cochlea after aminoglycoside-induced damage. Neurosci Lett. 2003;347(3):171–4. . - PubMed
-
- Ruben RJ. Development of the inner ear of the mouse: a radioautographic study of terminal mitoses. Acta Otolaryngol. 1967:Suppl 220:1–44. . - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

