EGFR transactivation contributes to neuroinflammation in Streptococcus suis meningitis
- PMID: 27756321
- PMCID: PMC5070219
- DOI: 10.1186/s12974-016-0734-0
EGFR transactivation contributes to neuroinflammation in Streptococcus suis meningitis
Abstract
Background: Streptococcus suis serotype 2 (SS2) is an important zoonotic bacterial pathogen in both humans and animals, which can cause high morbidity and mortality. Meningitis is one of the major clinical manifestations of SS2 infection. However, the specific process of SS2 meningitis and its molecular mechanisms remain unclear. Epidermal growth factor receptor (EGFR) has been reported to initiate transduction of intracellular signals and regulate host inflammatory responses. Whether and how EGFR contributes to the development of S. suis meningitis are currently unknown.
Methods: The tyrosine phosphorylation of cellular proteins, the transactivation of EGFR, as well as its dimerization, and the associated signal transduction pathways were investigated by immunoprecipitation and western blotting. Real-time quantitative PCR was used to investigate the transcriptional level of the ErbB family members, EGFR-related ligands, cytokines, and chemokines. The secretion of cytokines and chemokines in the serum and brain were detected by Q-Plex™ Chemiluminescent ELISA.
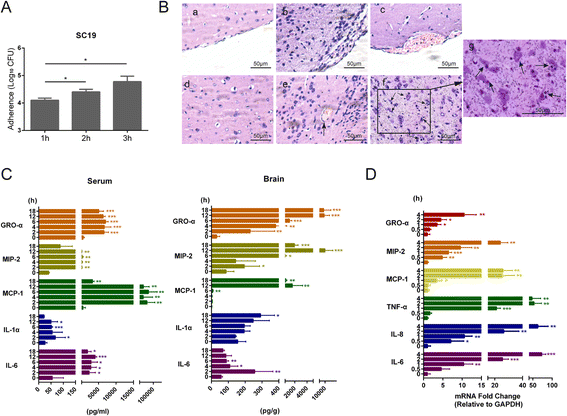
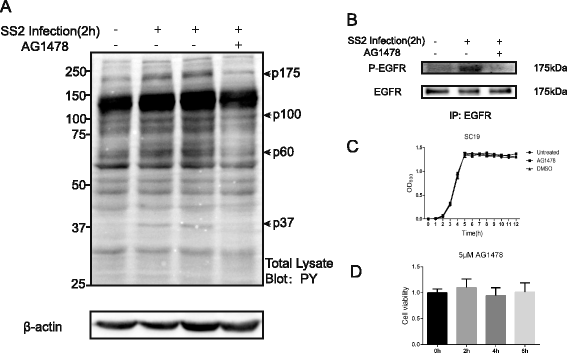
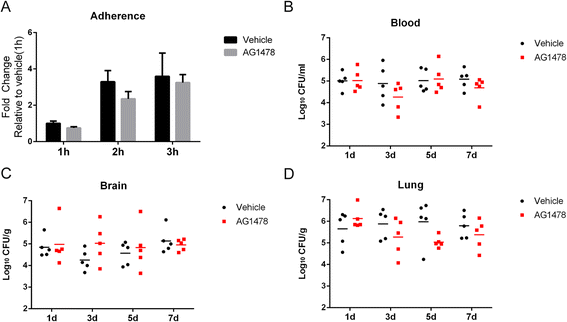
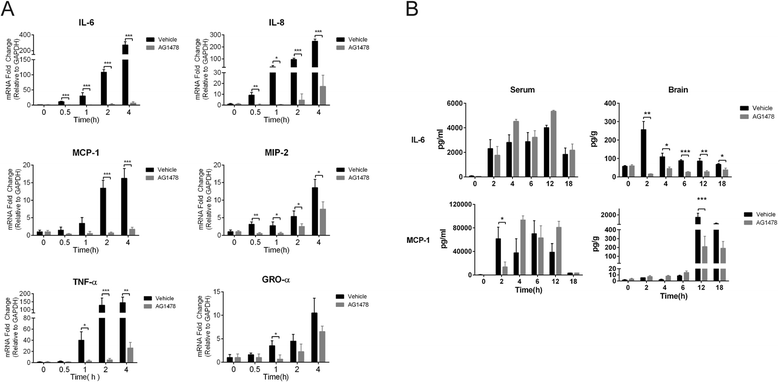
Results: We found an important role of EGFR in SS2 strain SC19-induced meningitis. SC19 increasingly adhered to human brain microvascular endothelial cells (hBMEC) and caused inflammatory lesions in the brain tissues, with significant induction and secretion of proinflammatory cytokines and chemokines in the serum and brains. SC19 infection of hBMEC induced tyrosine phosphorylation of cellular EGFR in a ligand-dependent manner involving the EGF-like ligand HB-EGF, amphiregulin (AREG), and epiregulin (EREG) and led to heterodimerization of EGFR/ErbB3. The EGFR transactivation did not participate in SS2 strain SC19 adhesion of hBMEC, as well as in bacterial colonization in vivo. However, its transactivation contributed to the bacterial-induced neuroinflammation, via triggering the MAPK-ERK1/2 and NF-κB signaling pathways in hBMEC that promote the production of proinflammatory cytokines and chemokines.
Conclusions: We investigated for the first time the tyrosine phosphorylation of cellular proteins in response to SS2 strain SC19 infection of hBMEC and demonstrated the contribution of EGFR to SS2-induced neuroinflammation. These observations propose a novel mechanism involving EGFR in SS2-mediated inflammatory responses in the brain, and therefore, EGFR might be an important host target for further investigation and prevention of neuroinflammation caused by SS2 strains.
Keywords: Brain microvascular endothelial cell; EGFR; Neuroinflammation; Streptococcus suis.
Figures


Similar articles
-
The VraSR two-component signal transduction system contributes to the damage of blood-brain barrier during Streptococcus suis meningitis.Microb Pathog. 2022 Nov;172:105766. doi: 10.1016/j.micpath.2022.105766. Epub 2022 Sep 7. Microb Pathog. 2022. PMID: 36087689
-
Host-pathogen Interaction at the Intestinal Mucosa Correlates With Zoonotic Potential of Streptococcus suis.J Infect Dis. 2015 Jul 1;212(1):95-105. doi: 10.1093/infdis/jiu813. Epub 2014 Dec 18. J Infect Dis. 2015. PMID: 25525050 Free PMC article.
-
Luteinizing hormone signaling in preovulatory follicles involves early activation of the epidermal growth factor receptor pathway.Mol Endocrinol. 2008 Apr;22(4):924-36. doi: 10.1210/me.2007-0246. Epub 2008 Jan 10. Mol Endocrinol. 2008. PMID: 18187604 Free PMC article.
-
How Streptococcus suis serotype 2 attempts to avoid attack by host immune defenses.J Microbiol Immunol Infect. 2019 Aug;52(4):516-525. doi: 10.1016/j.jmii.2019.03.003. Epub 2019 Mar 27. J Microbiol Immunol Infect. 2019. PMID: 30954397 Review.
-
Bacterial and host factors involved in zoonotic Streptococcal meningitis.Microbes Infect. 2025 Jan;27(1):105335. doi: 10.1016/j.micinf.2024.105335. Epub 2024 Apr 4. Microbes Infect. 2025. PMID: 38582147 Review.
Cited by
-
Polymorphism in the EREG gene confers susceptibility to tuberculosis.BMC Med Genet. 2019 Jan 11;20(1):7. doi: 10.1186/s12881-018-0729-z. BMC Med Genet. 2019. PMID: 30634928 Free PMC article.
-
miR-155 and miR-146a collectively regulate meningitic Escherichia coli infection-mediated neuroinflammatory responses.J Neuroinflammation. 2021 May 13;18(1):114. doi: 10.1186/s12974-021-02165-4. J Neuroinflammation. 2021. PMID: 33985523 Free PMC article.
-
Depletion of EREG enhances the osteo/dentinogenic differentiation ability of dental pulp stem cells via the p38 MAPK and Erk pathways in an inflammatory microenvironment.BMC Oral Health. 2021 Jun 21;21(1):314. doi: 10.1186/s12903-021-01675-0. BMC Oral Health. 2021. PMID: 34154572 Free PMC article.
-
Human rhomboid family-1 modulates clathrin coated vesicle-dependent pro-transforming growth factor α membrane trafficking to promote breast cancer progression.EBioMedicine. 2018 Oct;36:229-240. doi: 10.1016/j.ebiom.2018.09.038. Epub 2018 Sep 29. EBioMedicine. 2018. PMID: 30279141 Free PMC article.
-
Regulative synthesis of capsular polysaccharides in the pathogenesis of Streptococcus suis.Elife. 2025 Jun 23;13:RP101760. doi: 10.7554/eLife.101760. Elife. 2025. PMID: 40548692 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

