Efficacy and safety of angiogenesis inhibitors in advanced gastric cancer: a systematic review and meta-analysis
- PMID: 27756337
- PMCID: PMC5070169
- DOI: 10.1186/s13045-016-0340-8
Efficacy and safety of angiogenesis inhibitors in advanced gastric cancer: a systematic review and meta-analysis
Abstract
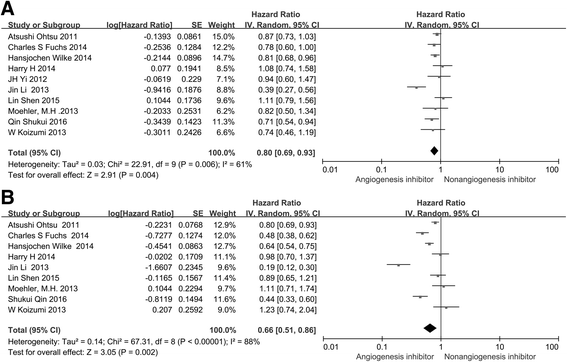
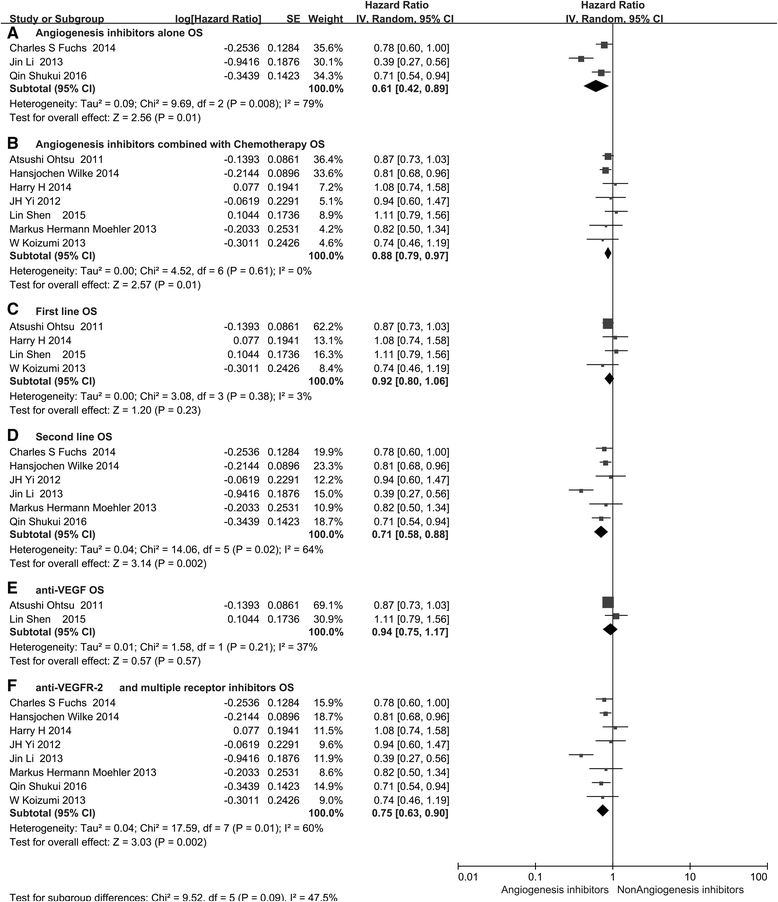
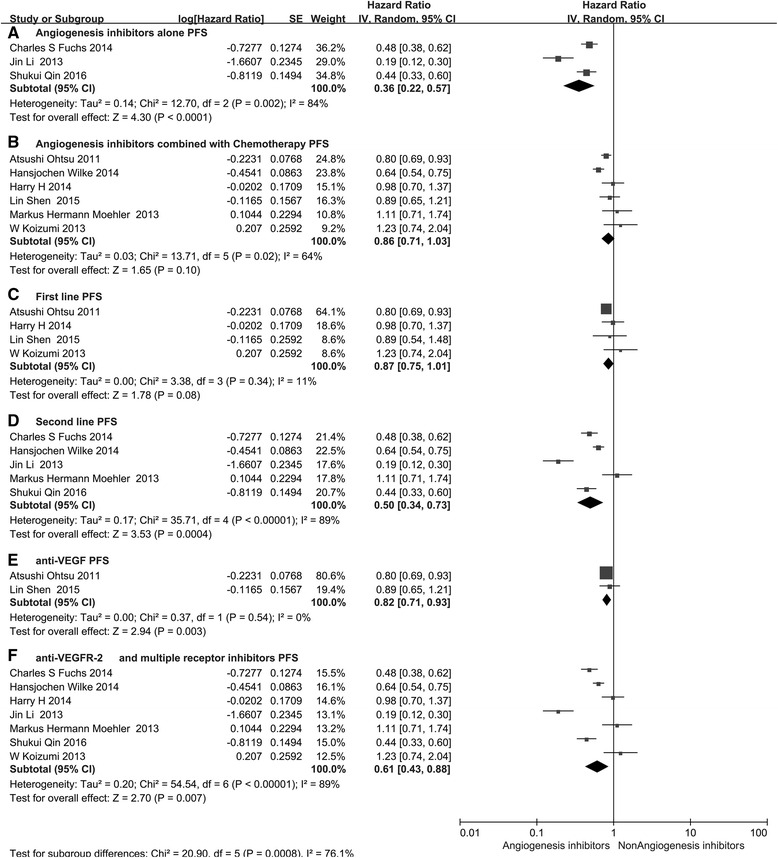
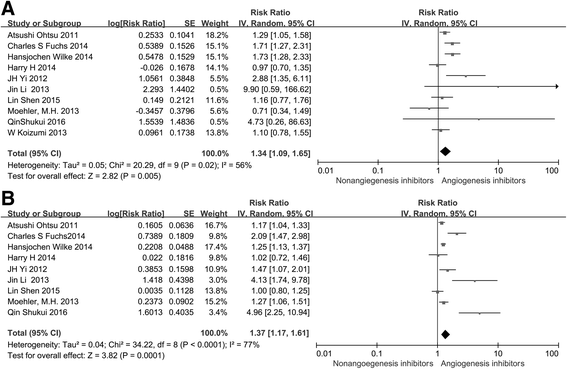
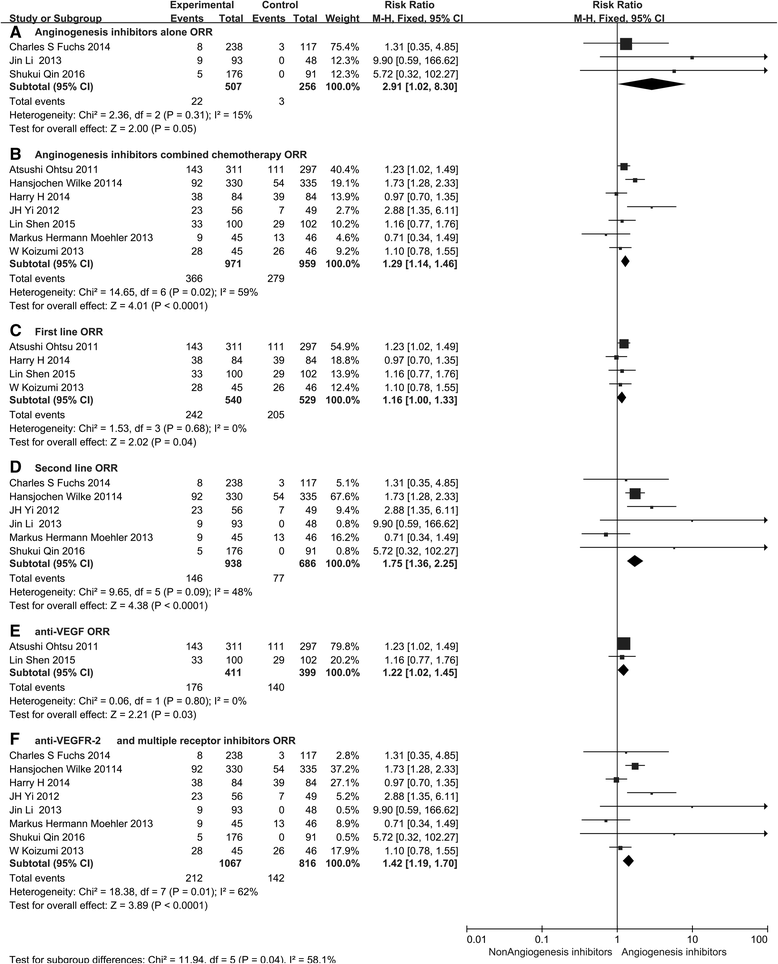
Monoclonal antibodies and small molecule tyrosine kinase inhibitors (TKIs) directed against the vascular endothelial growth factor (VEGF) or its receptors have been investigated in several studies for the treatment of advanced gastric cancer (GC). In the present study, we aimed to evaluate the efficacy and safety of angiogenesis inhibitors in advanced GC. We searched published randomized controlled trials (RCTs) comparing angiogenesis inhibitors with non-angiogenesis inhibitors for the treatment of GC. MEDLINE, EMBASE, and the Cochrane Controlled Trials Register were searched. The extracted data on progression-free survival (PFS) and overall survival (OS) were measured in terms of hazard ratios (HR) and corresponding 95 % confidence intervals (CIs). In addition, risk ratios (RR) and corresponding 95 % CIs were pooled for objective response rate (ORR), disease control rate (DCR), and risk of adverse events (AEs). Ten RCTs involving 2786 patients were included. Compared with non-angiogenesis inhibitor-containing regimens, angiogenesis inhibitor-containing regimens resulted in a significant improvement in OS (HR 0.80, 95 % CI 0.69-0.93, P = 0.004), prolonged PFS (HR 0.66, 95 % CI 0.51-0.86, P = 0.002), and superior ORR (RR 1.34, 95 % CI 1.09-1.65, P = 0.005) and DCR (RR 1.37, 95 % CI 1.17-1.61, P = 0.0001). Angiogenesis inhibitors were associated with a greater number of AEs, but most of these were predictable and manageable. However, hand-foot syndrome, diarrhea, and gastrointestinal (GI) perforation were significantly increased in patients treated with angiogenesis inhibitors. In summary, angiogenesis inhibitor-containing regimens were superior to non-angiogenesis inhibitor-containing regimens in terms of OS, PFS, RR, and DCR in patients with advanced GC.
Keywords: Angiogenesis; Gastric cancer; Monoclonal antibodies; Tyrosine kinase inhibitors.
Figures


References
-
- Sultana N: Globocan 2012: Estimated Cancer Incidence, Mortality and Prevalence Worldwide in 2012. Available from http://globocan.iarc.fr/Default.aspx. Accessed on 31 Aug. 2014.
-
- Lieto E, Ferraraccio F, Orditura M, Castellano P, Mura AL, Pinto M, Zamboli A, Vita FD, Galizia G. Expression of Vascular Endothelial Growth Factor (VEGF) and Epidermal Growth Factor Receptor (EGFR) is an Independent Prognostic Indicator of Worse Outcome in Gastric Cancer Patients. Ann Surg Oncol. 2007;15:69–79. doi: 10.1245/s10434-007-9596-0. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

