Dose-response effect of berberine on bile acid profile and gut microbiota in mice
- PMID: 27756364
- PMCID: PMC5070223
- DOI: 10.1186/s12906-016-1367-7
Dose-response effect of berberine on bile acid profile and gut microbiota in mice
Abstract
Background: Berberine (BBR) is a traditional antimicrobial herbal medicine. Recently, BBR has gained popularity as a supplement to lower blood lipids, cholesterol and glucose. Bile acids (BAs) are known to regulate blood levels of triglycerides, cholesterol, glucose and energy homeostasis, and gut flora play an important role in BA metabolism. However, whether BBR alters BAs metabolism or dose-response effect of BBR on gut flora is unknown.
Methods: In this study, the effects of various doses of BBR on the concentrations of BAs in liver and serum of male C57BL/6 mice were determined by UPLC-MS/MS, and the expression of BA-related genes, as well as the amount of 32 of the most abundant gut bacterial species in the terminal ileum and large intestine of male C57BL/6 mice were quantified by RT-PCR and Quantigene 2.0 Reagent System, respectively.
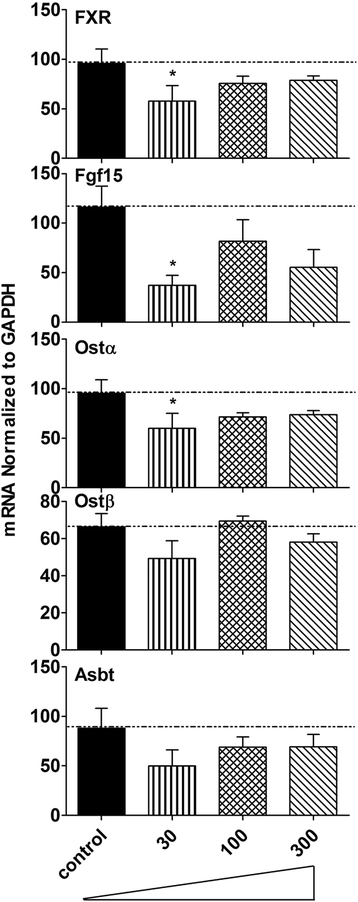
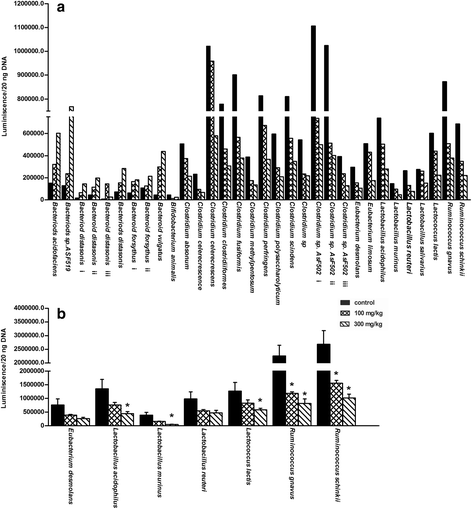
Results: Unconjugated BAs and total BAs were significantly altered by BBR in serum but not in liver. Increased primary BAs (βMCA, TβMCA and TUDCA) and decreased secondary BAs (DCA, LCA and the T-conjugates) were observed in livers and serum of mice fed BBR. The expression of BA-synthetic enzymes (Cyp7a1 and 8b1) and uptake transporter (Ntcp) increased 39-400 % in liver of mice fed the higher doses of BBR, whereas nuclear receptors and efflux transporters were not markedly altered. In addition, Bacteroides were enriched in the terminal ileum and large bowel of mice treated with BBR.
Conclusion: The present study indicated that various doses of BBR have effects on BA metabolism and related genes as well as intestinal flora, which provides insight into many pathways of BBR effects.
Keywords: Berberine; Bile acids; Gut Microbiota; Mice.
Figures





References
-
- Fiorucci S, Cipriani S, Mencarelli A, Renga B, Distrutti E, Baldelli F. Counter-regulatory role of bile acid activated receptors in immunity and inflammation. Curr Mol Med. 2010;10(6):579–95. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

