Spatio-temporal mutation profiles of case-matched colorectal carcinomas and their metastases reveal unique de novo mutations in metachronous lung metastases by targeted next generation sequencing
- PMID: 27756406
- PMCID: PMC5069823
- DOI: 10.1186/s12943-016-0549-8
Spatio-temporal mutation profiles of case-matched colorectal carcinomas and their metastases reveal unique de novo mutations in metachronous lung metastases by targeted next generation sequencing
Abstract
Background: Targeted next generation sequencing (tNGS) has become part of molecular pathology diagnostics for determining RAS mutation status in colorectal cancer (CRC) patients as predictive tool for decision on EGFR-targeted therapy. Here, we investigated mutation profiles of case-matched tissue specimens throughout the disease course of CRC, to further specify RAS-status dynamics and to identify de novo mutations associated with distant metastases.
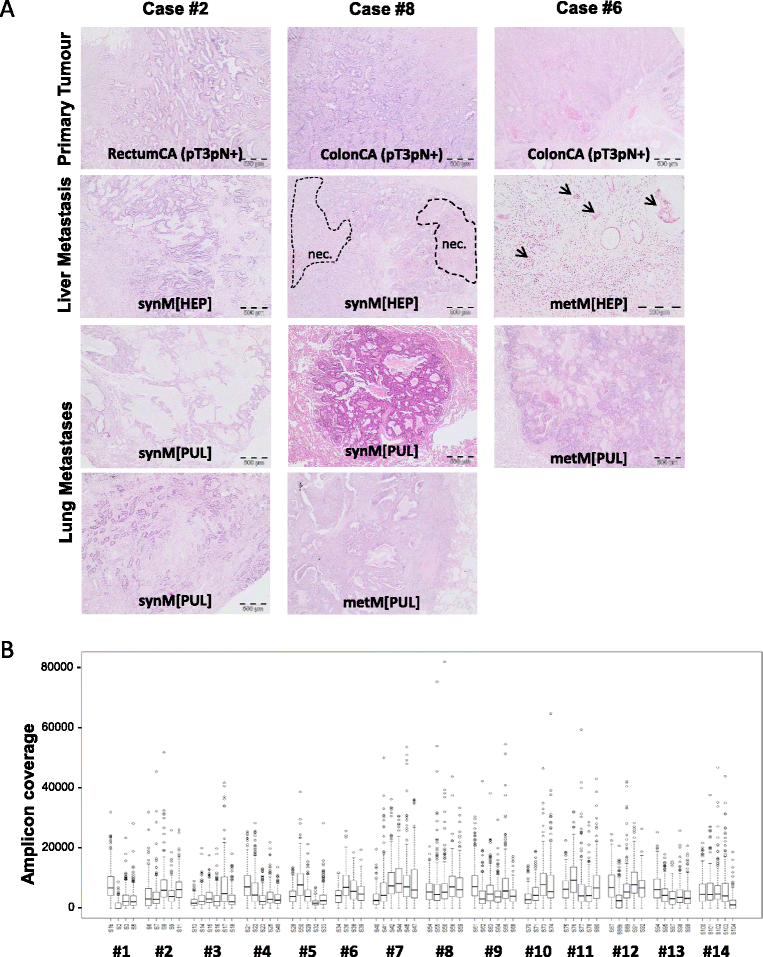
Methods: Case-matched formalin-fixed and paraffin-embedded (FFPE) resection specimens (n = 70; primary tumours, synchronous and/or metachronous liver and/or lung metastases) of 14 CRC cases were subjected to microdissection of normal colonic epithelial, primary and metastatic tumour cells, their DNA extraction and an adapted library protocol for limited DNA using the 48 gene TruSeq Amplicon Cancer PanelTM, MiSeq sequencing and data analyses (Illumina).
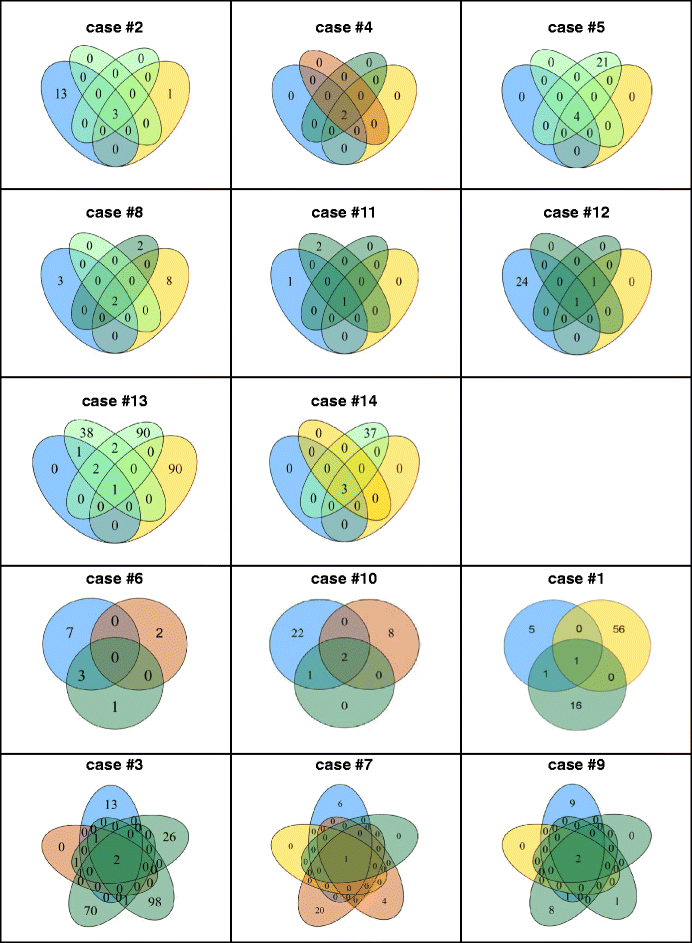
Results: By tNGS primary tumours were RAS wildtype in 5/14 and mutated in 9/14 (8/9 KRAS exon 2; 1/9 NRAS Exon 3) of cases. RAS mutation status was maintained in case-matched metastases throughout the disease course, albeit with altered allele frequencies. Case-matched analyses further identified a maximum of three sequence variants (mainly in APC, KRAS, NRAS, TP53) shared by all tumour specimens throughout the disease course per individual case. In addition, further case-matched de novo mutations were detected in synchronous and/or metachronous liver and/or lung metastases (e.g. in APC, ATM, FBXW7, FGFR3, GNAQ, KIT, PIK3CA, PTEN, SMAD4, SMO, STK11, TP53, VHL). Moreover, several de novo mutations were more frequent in synchronous (e.g. ATM, KIT, PIK3CA, SMAD4) or metachronous (e.g. FBXW7, SMO, STK11) lung metastases. Finally, some de novo mutations occurred only in metachronous lung metastases (CDKN2A, FGFR2, GNAS, JAK3, SRC).
Conclusion: Together, this study employs an adapted FFPE-based tNGS approach to confirm conservation of RAS mutation status in primary and metastatic tissue specimens of CRC patients. Moreover, it identifies genes preferentially mutated de novo in late disease stages of metachronous CRC lung metastases, several of which might be actionable by targeted therapies.
Keywords: Colorectal cancer; FFPE; Metastases; Next generation sequencing.
Figures



References
-
- Schwartzberg LS, Rivera F, Karthaus M, et al. PEAK: a randomized, multicenter phase II study of panitumumab plus modified fluorouracil, leucovorin, and oxaliplatin (mFOLFOX6) or bevacizumab plus mFOLFOX6 in patients with previously untreated, unresectable, wild-type KRAS exon 2 metastatic colorectal cancer. J Clin Oncol. 2014;32:2240–2247. doi: 10.1200/JCO.2013.53.2473. - DOI - PubMed
-
- Ashraf N, Kothari N, Kim R. Predictive biomarkers for anti-epidermal growth factor receptor therapy: beyond KRAS testing. J Natl Compr Canc Netw. 2014;12:1433–1442. - PubMed
-
- Modest DP, Stintzing S, von Weikersthal LF, et al. Impact of Subsequent Therapies on Outcome of the FIRE-3/AIO KRK0306 Trial: First-Line Therapy With FOLFIRI Plus Cetuximab or Bevacizumab in Patients With KRAS Wild-Type Tumors in Metastatic Colorectal Cancer. J Clin Oncol. 2015;33:3718–3726. doi: 10.1200/JCO.2015.61.2887. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

