ABT-126 monotherapy in mild-to-moderate Alzheimer's dementia: randomized double-blind, placebo and active controlled adaptive trial and open-label extension
- PMID: 27756421
- PMCID: PMC5067903
- DOI: 10.1186/s13195-016-0210-1
ABT-126 monotherapy in mild-to-moderate Alzheimer's dementia: randomized double-blind, placebo and active controlled adaptive trial and open-label extension
Abstract
Background: Results from a phase 2a study indicated that treatment with the novel α7 nicotinic acetylcholine receptor agonist ABT-126 25 mg once daily (QD) was associated with a trend for improvement in cognition in subjects with mild-to-moderate Alzheimer's dementia (AD). A phase 2b program was designed to evaluate a broader dose range of ABT-126 as monotherapy in subjects with mild-to-moderate AD. The program consisted of a double-blind, placebo and active controlled study of ABT-126 (dose range 25-75 mg) and an open-label extension study (75 mg).
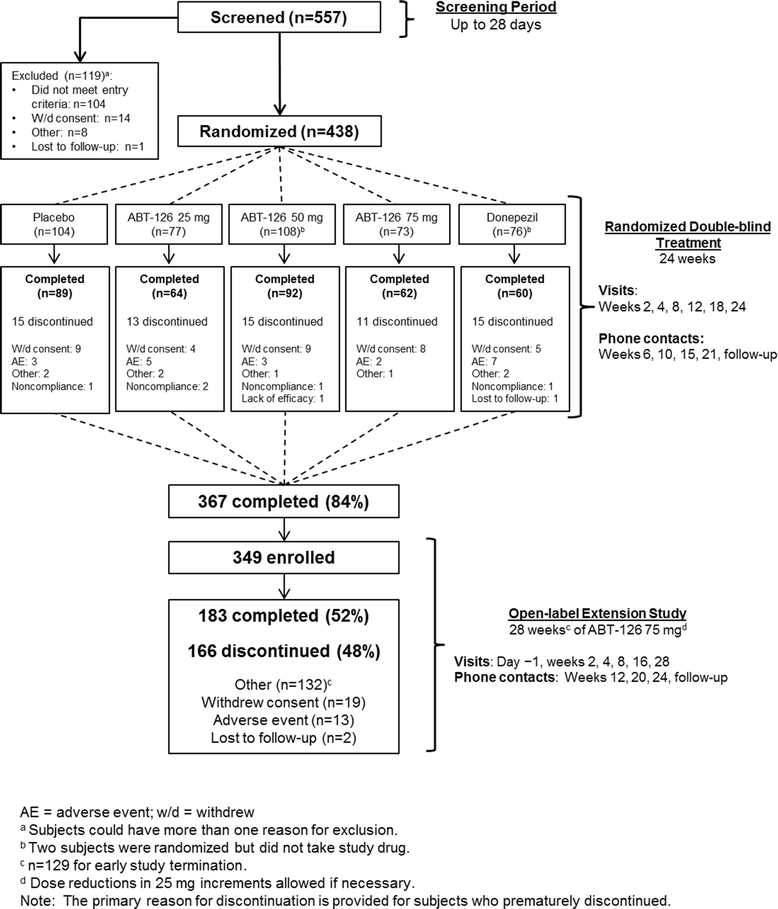
Methods: The randomized double-blind study enrolled 438 subjects (Mini-Mental Status Examination score of 10-24, inclusive) not currently taking acetylcholinesterase inhibitors or memantine. Subjects received 24 weeks of ABT-126 25 mg QD (n = 77), ABT-126 50 mg QD (n = 108), ABT-126 75 mg QD (n = 73), donepezil 10 mg QD (n = 76), or placebo (n = 104). The primary endpoint was the change from baseline to week 24 in the 11-item Alzheimer's Disease Assessment Scale-Cognitive subscale (ADAS-Cog) total score. Subjects completing the double-blind study could enroll in the 28-week open-label extension study. Adverse events (AEs) and other safety parameters were monitored in both studies.
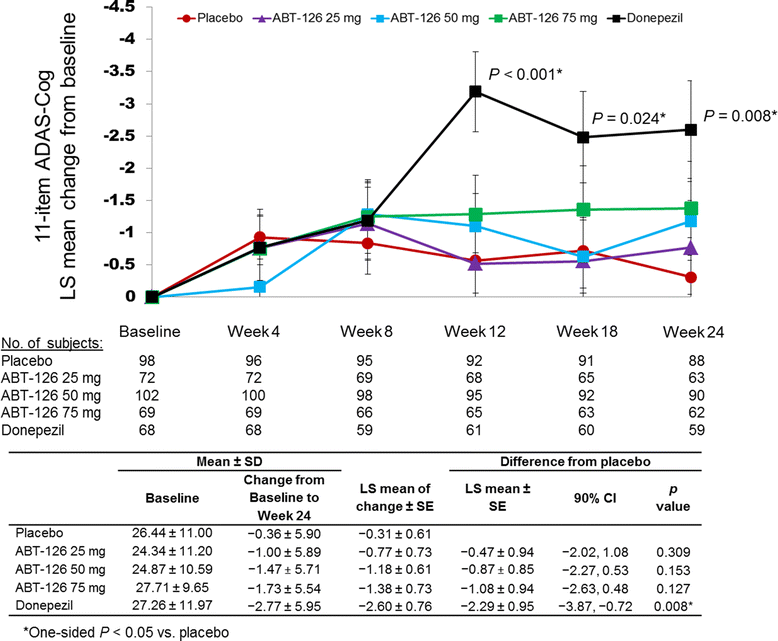
Results: A total of 367 patients (83.8 %) completed the double-blind study and 349 (79.7 %) entered the open-label study. Compared with placebo, donepezil significantly improved ADAS-Cog 11-item total scores from baseline to week 24 (-2.29 ± 0.95; one-sided P = 0.008). No ABT-126 dose demonstrated a statistically significant improvement vs placebo at week 24 in the ADAS-Cog total score: ABT-126 25 mg, -0.47 ± 0.94 (P = 0.309); ABT-126 50 mg, -0.87 ± 0.85 (P = 0.153); and ABT-126 75 mg, -1.08 ± 0.94 (P = 0.127). Rates of serious AEs and discontinuations due to AEs were similar across treatment groups. The most frequently reported AEs in both studies were constipation, fall, and headache. No clinically meaningful changes were observed in other parameters.
Conclusions: In the double-blind trial, donepezil significantly improved ADAS-Cog scores but no statistically significant improvement was seen with any ABT-126 dose. ABT-126 had an acceptable safety profile in subjects with mild-to-moderate AD in both studies.
Trial registration: ClinicalTrials.gov NCT01527916 , Registered 3 February 2012 (randomized trial). ClinicalTrials.gov NCT01676935 . Registered 29 August 2012 (open-label extension study).
Keywords: ABT-126; Adaptive design; Alzheimer’s dementia/drug therapy; Alzheimer’s disease; Assessment of cognitive disorders/dementia; Nicotinic agonists; Randomized controlled trial.
Figures
Similar articles
-
Efficacy and Safety of ABT-126 in Subjects with Mild-to-Moderate Alzheimer's Disease on Stable Doses of Acetylcholinesterase Inhibitors: A Randomized, Double-Blind, Placebo-Controlled Study.J Alzheimers Dis. 2016;51(4):1237-47. doi: 10.3233/JAD-150978. J Alzheimers Dis. 2016. PMID: 26967214 Clinical Trial.
-
A Phase 2 clinical trial of PF-05212377 (SAM-760) in subjects with mild to moderate Alzheimer's disease with existing neuropsychiatric symptoms on a stable daily dose of donepezil.Alzheimers Res Ther. 2018 Apr 5;10(1):38. doi: 10.1186/s13195-018-0368-9. Alzheimers Res Ther. 2018. PMID: 29622037 Free PMC article. Clinical Trial.
-
Donepezil improves cognition and global function in Alzheimer disease: a 15-week, double-blind, placebo-controlled study. Donepezil Study Group.Arch Intern Med. 1998 May 11;158(9):1021-31. doi: 10.1001/archinte.158.9.1021. Arch Intern Med. 1998. PMID: 9588436 Clinical Trial.
-
Perspectives in the management of Alzheimer's disease: clinical profile of donepezil.Dement Geriatr Cogn Disord. 1998;9 Suppl 3:29-42. doi: 10.1159/000051201. Dement Geriatr Cogn Disord. 1998. PMID: 9853200 Review.
-
Donepezil and rivastigmine in the treatment of Alzheimer's disease: a best-evidence synthesis of the published data on their efficacy and cost-effectiveness.Clin Ther. 2002 Jun;24(6):862-86; discussion 837. doi: 10.1016/s0149-2918(02)80004-2. Clin Ther. 2002. PMID: 12117079 Review.
Cited by
-
Evaluating the efficacy and safety of Alzheimer's disease drugs: A meta-analysis and systematic review.Medicine (Baltimore). 2024 Apr 19;103(16):e37799. doi: 10.1097/MD.0000000000037799. Medicine (Baltimore). 2024. PMID: 38640313 Free PMC article.
-
Therapeutic Targeting of the α7 Nicotinic Receptor: Challenges and Prospects for Cognitive Improvement in Alzheimer's and Schizophrenia.Basic Clin Pharmacol Toxicol. 2025 Jul;137(1):e70061. doi: 10.1111/bcpt.70061. Basic Clin Pharmacol Toxicol. 2025. PMID: 40456556 Free PMC article. Review.
-
Diagnostic and inclusion criteria in Alzheimer's disease clinical trials: A systematic review of the past decade.J Alzheimers Dis Rep. 2025 Jul 30;9:25424823251362444. doi: 10.1177/25424823251362444. eCollection 2025 Jan-Dec. J Alzheimers Dis Rep. 2025. PMID: 40755858 Free PMC article. Review.
-
A Systematic Review on Drugs Acting as Nicotinic Acetylcholine Receptor Agonists in the Treatment of Dementia.Cells. 2024 Jan 26;13(3):237. doi: 10.3390/cells13030237. Cells. 2024. PMID: 38334629 Free PMC article.
-
The current agonists and positive allosteric modulators of α7 nAChR for CNS indications in clinical trials.Acta Pharm Sin B. 2017 Nov;7(6):611-622. doi: 10.1016/j.apsb.2017.09.001. Epub 2017 Oct 16. Acta Pharm Sin B. 2017. PMID: 29159020 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

