RARγ-induced E-cadherin downregulation promotes hepatocellular carcinoma invasion and metastasis
- PMID: 27756432
- PMCID: PMC5069892
- DOI: 10.1186/s13046-016-0441-9
RARγ-induced E-cadherin downregulation promotes hepatocellular carcinoma invasion and metastasis
Abstract
Background: Aberrant expression of Retinoic acid receptor γ (RARγ) is implicated in cancer development. Our previous study identified that RARγ functions as a tumor promoter to drive hepatocellular carcinoma (HCC) growth. However, its contribution to HCC invasion and metastasis remains unclear.
Methods: RARγ expression in clinical HCC samples was detected by western blot and immunohistochemistry. The relationship between RARγ expression levels and the clinical characteristics were evaluated. HCC cell line MHCC-97H were stably knocked down RARγ using a lentivirus vector-based shRNA technique. The cells were analyzed by migration and invasion assays, and injected into nude mice to assess tumor metastasis. E-cadherin expression regulated by RARγ was examined by qPCR, western blot and immunofluorescence staining.
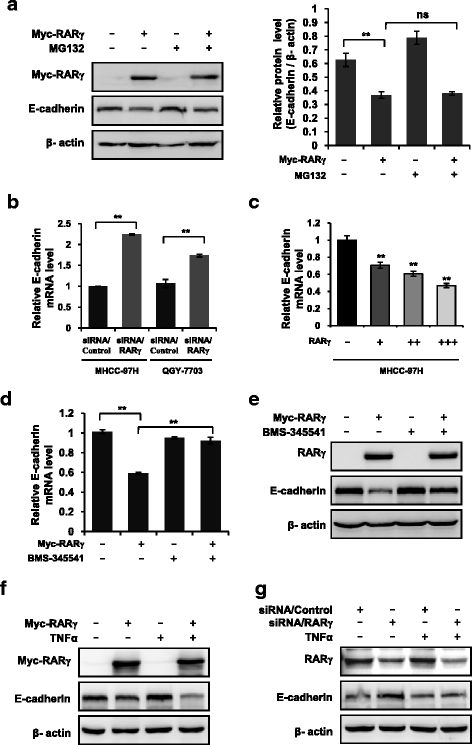
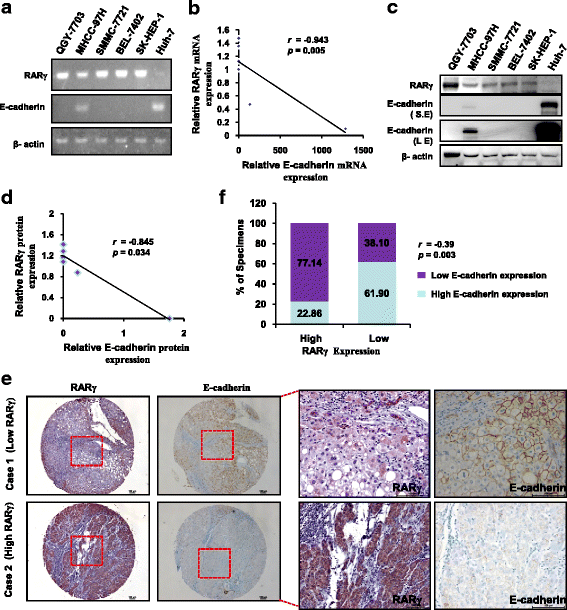
Results: The expression of RARγ is significantly upregulated in human HCC tissues. Moreover, its expression positively correlates with tumor size, distant metastasis and TNM stage, and negatively correlates with length of survival of HCC patients. Knockdown of RARγ markedly inhibits HCC cell invasion and metastasis both in vitro and in vivo. Mechanistic investigations reveal that RARγ functions through regulation of NF-κB-mediated E-cadherin downregulation to promote HCC invasion and metastasis. Notably, RARγ expression status negatively correlates with E-cadherin expression in HCC cell lines and clinical HCC samples.
Conclusions: These findings demonstrate that RARγ could promote HCC invasion and metastasis by regulating E-cadherin reduction, and implicate new strategies to aggressively treat HCC through targeting RARγ/E-cadherin signaling axis.
Keywords: E-cadherin; Hepatocellular carcinoma; Metastasis; RARγ.
Figures



References
-
- Yeo W, Mok TS, Zee B, Leung TW, Lai PB, Lau WY, Koh J, Mo FK, Yu SC, Chan AT, et al. A randomized phase III study of doxorubicin versus cisplatin/interferon alpha-2b/doxorubicin/fluorouracil (PIAF) combination chemotherapy for unresectable hepatocellular carcinoma. J Natl Cancer Inst. 2005;97(20):1532–1538. doi: 10.1093/jnci/dji315. - DOI - PubMed
-
- Du ZG, Wei YG, Chen KF, Li B. Risk factors associated with early and late recurrence after curative resection of hepatocellular carcinoma: a single institution's experience with 398 consecutive patients. Hepatobiliary Pancreat Dis Int. 2014;13(2):153–161. doi: 10.1016/S1499-3872(14)60025-4. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

