Protective effect of 3-(naphthalen-2-yl(propoxy)methyl)azetidine hydrochloride on hypoxia-induced toxicity by suppressing microglial activation in BV-2 cells
- PMID: 27756444
- PMCID: PMC5346314
- DOI: 10.5483/bmbrep.2016.49.12.169
Protective effect of 3-(naphthalen-2-yl(propoxy)methyl)azetidine hydrochloride on hypoxia-induced toxicity by suppressing microglial activation in BV-2 cells
Abstract
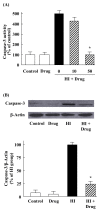
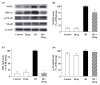
We recently reported the anti-inflammatory effects of 3-(naphthalen-2-yl(propoxy)methyl)azetidine hydrochloride (KHG26792) on the ATP-induced activation of the NFAT and MAPK pathways through the P2X7 receptor in microglia. To further investigate the underlying mechanism of KHG26792, we studied its protective effects on hypoxia-induced toxicity in microglia. The administration of KHG26792 significantly reduced the hypoxia-induced expression and activity of caspase-3 in BV-2 microglial cells. KHG26792 also reduced hypoxia-induced inducible nitric oxide synthase protein expression, which correlated with reduced nitric oxide accumulation. In addition, KHG26792 attenuated hypoxiainduced protein nitration, reactive oxygen species production, and NADPH oxidase activity. These effects were accompanied by the suppression of hypoxia-induced protein expression of hypoxia-inducible factor 1-alpha and NADPH oxidase-2. Although the clinical relevance of our findings remains to be determined, these data results suggest that KHG26792 prevents hypoxia-induced toxicity by suppressing microglial activation. [BMB Reports 2016; 49(12): 687-692].
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

