Effect of smokeless tobacco products on human oral bacteria growth and viability
- PMID: 27756619
- PMCID: PMC5693344
- DOI: 10.1016/j.anaerobe.2016.10.006
Effect of smokeless tobacco products on human oral bacteria growth and viability
Abstract
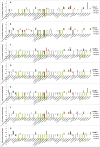
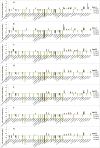
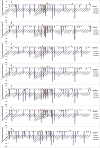
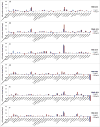
To evaluate the toxicity of smokeless tobacco products (STPs) on oral bacteria, seven smokeless tobacco aqueous extracts (STAEs) from major brands of STPs and three tobacco-specific N-nitrosamines (TSNAs) were used in a growth and viability test against 38 oral bacterial species or subspecies. All seven STAEs showed concentration-dependent effects on the growth and viability of tested oral bacteria under anaerobic culture conditions, although there were strain-to-strain variations. In the presence of 1 mg/ml STAEs, the growth of 4 strains decreased over 0.32-2.14 log10 fold, while 14 strains demonstrated enhanced growth of 0.3-1.76 log10 fold, and the growth of 21 strains was not significantly affected. In the presence of 10 mg/ml STAEs, the growth of 17 strains was inhibited 0.3-2.11 log10 fold, 18 strains showed enhanced growth of 0.3-0.97 log10 fold, and 4 strains were not significantly affected. In the presence of 50 mg/ml STAEs, the growth of 32 strains was inhibited 0.3-2.96 log10 fold, 8 strains showed enhanced growth of 0.3-1.0 log10 fold, and 2 strains were not significantly affected. All seven STAEs could promote the growth of 4 bacterial strains, including Eubacterium nodatum, Peptostreptococcus micros, Streptococcus anginosus, and Streptococcus constellatus. Exposure to STAEs modulated the viability of some bacterial strains, with 21.1-66.5% decrease for 4 strains at 1 mg/ml, 20.3-85.7% decrease for 10 strains at 10 mg/ml, 20.0-93.3% decrease for 27 strains at 50 mg/ml, and no significant effect for 11 strains at up to 50 mg/ml. STAEs from snuffs inhibited more tested bacterial strains than those from snus indicating that the snuffs may be more toxic to the oral bacteria than snus. For TSNAs, cell growth and viability of 34 tested strains were not significantly affected at up to 100 μg/ml; while the growth of P. micros was enhanced 0.31-0.54 log10 fold; the growth of Veillonella parvula was repressed 0.33-0.36 log10 fold; and the cell viabilities of 2 strains decreased 56.6-69.9%. The results demonstrate that STAEs affected the growth of some types of oral bacteria, which may affect the healthy ecological balance of oral bacteria in humans. On the other hand, TSNAs did not significantly affect the growth of the oral bacteria.
Keywords: Cell viability; Oral bacteria; Smokeless tobacco; Tobacco-specific N-nitrosamines; Toxicology.
Published by Elsevier Ltd.
Figures

Similar articles
-
Smokeless Tobacco Products (STPs) Harbour Bacterial Populations with Potential for Oral Carcinogenicity.Asian Pac J Cancer Prev. 2020 Mar 1;21(3):815-824. doi: 10.31557/APJCP.2020.21.3.815. Asian Pac J Cancer Prev. 2020. PMID: 32212812 Free PMC article.
-
Composition and Ecological Functionality of Fungal Communities Associated with Smokeless Tobacco Products Mainly Consumed in India.Microbiol Spectr. 2022 Aug 31;10(4):e0227321. doi: 10.1128/spectrum.02273-21. Epub 2022 Jun 13. Microbiol Spectr. 2022. PMID: 35695566 Free PMC article.
-
Microbial community and functions involved in smokeless tobacco product: a metagenomic approach.Appl Microbiol Biotechnol. 2024 Jun 25;108(1):395. doi: 10.1007/s00253-024-13156-9. Appl Microbiol Biotechnol. 2024. PMID: 38918238 Free PMC article.
-
Impact of smokeless tobacco-associated bacteriome in oral carcinogenesis.Anaerobe. 2021 Aug;70:102400. doi: 10.1016/j.anaerobe.2021.102400. Epub 2021 Jun 6. Anaerobe. 2021. PMID: 34090995 Review.
-
Microorganisms: crucial players of smokeless tobacco for several health attributes.Appl Microbiol Biotechnol. 2021 Aug;105(16-17):6123-6132. doi: 10.1007/s00253-021-11460-2. Epub 2021 Jul 31. Appl Microbiol Biotechnol. 2021. PMID: 34331556 Review.
Cited by
-
Smokeless Tobacco Harbors Bacteria Involved in Biofilm Formation as Well as Salt and Heavy Metal Tolerance Activity.Appl Biochem Biotechnol. 2024 Jun;196(6):3034-3055. doi: 10.1007/s12010-023-04689-2. Epub 2023 Aug 23. Appl Biochem Biotechnol. 2024. PMID: 37610514
-
Smokeless tobacco use and public health nutrition: a global systematic review.Public Health Nutr. 2023 Jan;26(1):46-55. doi: 10.1017/S1368980022001331. Epub 2022 May 27. Public Health Nutr. 2023. PMID: 35618706 Free PMC article.
-
Characterization of Oral Bacterial Composition of Adult Smokeless Tobacco Users from Healthy Indians Using 16S rDNA Analysis.Microb Ecol. 2021 Nov;82(4):1061-1073. doi: 10.1007/s00248-021-01711-0. Epub 2021 Feb 26. Microb Ecol. 2021. PMID: 33634334
-
Effect of different forms of tobacco on the oral microbiome in healthy adults: a systematic review.Front Oral Health. 2024 Feb 20;5:1310334. doi: 10.3389/froh.2024.1310334. eCollection 2024. Front Oral Health. 2024. PMID: 38445094 Free PMC article. Review.
-
Oral microbiome dysbiosis among cigarette smokers and smokeless tobacco users compared to non-users.Sci Rep. 2024 May 6;14(1):10394. doi: 10.1038/s41598-024-60730-2. Sci Rep. 2024. PMID: 38710815 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

