Developmental manganese neurotoxicity in rats: Cognitive deficits in allocentric and egocentric learning and memory
- PMID: 27756629
- PMCID: PMC5235975
- DOI: 10.1016/j.ntt.2016.10.005
Developmental manganese neurotoxicity in rats: Cognitive deficits in allocentric and egocentric learning and memory
Abstract
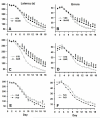
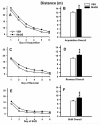
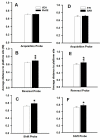
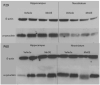
Manganese (Mn) is an essential element but neurotoxic at higher exposure levels. The effects of Mn overexposure (MnOE) on hippocampal and striatal-dependent learning and memory in rats were tested in combination with iron deficiency (FeD) and developmental stress that often co-occur with MnOE. Moderate FeD affects up to 15% of U.S. children and developmental stress is common in lower socio-economic areas where MnOE occurs. Pregnant Sprague-Dawley rats and their litters were housed in cages with or without (barren cage (BAR)) standard bedding from embryonic day (E)7 to postnatal day (P)28. Dams were fed a 90% FeD or iron sufficient (FeS) diet from E15-P28. Within each litter, separate offspring were treated with 100mg/kg Mn (MnOE) or vehicle (VEH) by gavage on alternate days from P4-28. Offspring were tested as adults in the Morris and Cincinnati water mazes. FeD and developmental stress interactively impaired spatial learning in the Morris water maze. Developmental stress and MnOE impaired learning and memory in both mazes. MnOE resulted in reduced CA1 hippocampal long-term potentiation (LTP) and increased levels of α-synuclein. Preweaning MnOE resulted in cognitive deficits on multiple domains of learning and memory accompanied by impaired LTP and α-synuclein changes, effects worsened by developmental stress.
Keywords: Alpha-synuclein; Egocentric learning; Long-term potentiation; Manganese; Neurotoxicity; Spatial learning.
Copyright © 2016 Elsevier Inc. All rights reserved.
Figures



References
-
- Amos-Kroohs RM, Bloor CP, Qureshi MA, Vorhees CV, Williams MT. Effects of developmental exposure to manganese and/or low iron diet: Changes to metal transporters, surcrose preference, elevated zero-maze, open-field, and locomotion in response to fenfluamine, amphetamine, and MK-801. Toxicol Rep. 2015;2:1046–1056. - PMC - PubMed
-
- Baier CJ, Katunar MR, Adrover E, Pallares ME, Antonelli MC. Gestational restraint stress and the developing dopaminergic system: an overview. Neurotox Res. 2012;22:16–32. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

