TGFβR1 Blockade with Galunisertib (LY2157299) Enhances Anti-Neuroblastoma Activity of the Anti-GD2 Antibody Dinutuximab (ch14.18) with Natural Killer Cells
- PMID: 27756784
- PMCID: PMC5361893
- DOI: 10.1158/1078-0432.CCR-16-1743
TGFβR1 Blockade with Galunisertib (LY2157299) Enhances Anti-Neuroblastoma Activity of the Anti-GD2 Antibody Dinutuximab (ch14.18) with Natural Killer Cells
Abstract
Purpose: Immunotherapy of high-risk neuroblastoma using the anti-GD2 antibody dinutuximab induces antibody-dependent cell-mediated cytotoxicity (ADCC). Galunisertib, an inhibitor of TGFβR1, was examined for its ability to enhance the efficacy of dinutuximab in combination with human ex vivo activated NK (aNK) cells against neuroblastoma.
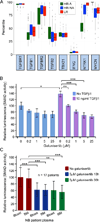
Experimental design: TGFB1 and TGFBR1 mRNA expression was determined for 249 primary neuroblastoma tumors by microarray analysis. The ability of galunisertib to inhibit SMAD activity induced by neuroblastoma patient blood and bone marrow plasmas in neuroblastoma cells was tested. The impact of galunisertib on TGFβ1-induced inhibition of aNK cytotoxicity and ADCC in vitro and on anti-neuroblastoma activity in NOD-scid gamma (NSG) mice was determined.
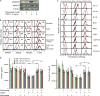
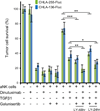
Results: Neuroblastomas express TGFB1 and TGFBR1 mRNA. Galunisertib suppressed SMAD activation in neuroblastoma cells induced by exogenous TGFβ1 or by patient blood and bone marrow plasma, and suppressed SMAD2 phosphorylation in human neuroblastoma cells growing in NSG mice. In NK cells treated in vitro with exogenous TGFβ1, galunisertib suppressed SMAD2 phosphorylation and restored the expression of DNAM-1, NKp30, and NKG2D cytotoxicity receptors and the TRAIL death ligand, the release of perforin and granzyme A, and the direct cytotoxicity and ADCC of aNK cells against neuroblastoma cells. Addition of galunisertib to adoptive cell therapy with aNK cells plus dinutuximab reduced tumor growth and increased survival of mice injected with two neuroblastoma cell lines or a patient-derived xenograft.
Conclusions: Galunisertib suppresses activation of SMAD2 in neuroblastomas and aNK cells, restores NK cytotoxic mechanisms, and increases the efficacy of dinutuximab with aNK cells against neuroblastoma tumors. Clin Cancer Res; 23(3); 804-13. ©2016 AACRSee related commentary by Zenarruzabeitia et al., p. 615.
©2016 American Association for Cancer Research.
Conflict of interest statement
Figures


Comment in
-
Natural Killer Cells to the Attack: Combination Therapy against Neuroblastoma.Clin Cancer Res. 2017 Feb 1;23(3):615-617. doi: 10.1158/1078-0432.CCR-16-2478. Epub 2016 Nov 21. Clin Cancer Res. 2017. PMID: 27872101
References
-
- Wilson EB, El-Jawhari JJ, Neilson AL, Hall GD, Melcher AA, Meade JL, et al. Human tumour immune evasion via TGF-beta blocks NK cell activation but not survival allowing therapeutic restoration of anti-tumour activity. PLoS One. 2011;6(9):e22842. PubMed PMID: 21909397; PMCID: PMC3167809. - PMC - PubMed
-
- Massagué J, Blain SW, Lo RS. TGFbeta signaling in growth control, cancer, and heritable disorders. Cell. 2000;103(2):295–309. PubMed PMID: 11057902. - PubMed
-
- Padua D, Massagué J. Roles of TGFbeta in metastasis. Cell Res. 2009;19(1):89–102. PubMed PMID: 19050696. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

