Control of Autophagy in Chlamydomonas Is Mediated through Redox-Dependent Inactivation of the ATG4 Protease
- PMID: 27756818
- PMCID: PMC5129734
- DOI: 10.1104/pp.16.01582
Control of Autophagy in Chlamydomonas Is Mediated through Redox-Dependent Inactivation of the ATG4 Protease
Abstract
Autophagy is a major catabolic pathway by which eukaryotic cells deliver unnecessary or damaged cytoplasmic material to the vacuole for its degradation and recycling in order to maintain cellular homeostasis. Control of autophagy has been associated with the production of reactive oxygen species in several organisms, including plants and algae, but the precise regulatory molecular mechanisms remain unclear. Here, we show that the ATG4 protease, an essential protein for autophagosome biogenesis, plays a central role for the redox regulation of autophagy in the model green alga Chlamydomonas reinhardtii Our results indicate that the activity of C. reinhardtii ATG4 is regulated by the formation of a single disulfide bond with a low redox potential that can be efficiently reduced by the NADPH/thioredoxin system. Moreover, we found that treatment of C. reinhardtii cells with norflurazon, an inhibitor of carotenoid biosynthesis that generates reactive oxygen species and triggers autophagy in this alga, promotes the oxidation and aggregation of ATG4. We propose that the activity of the ATG4 protease is finely regulated by the intracellular redox state, and it is inhibited under stress conditions to ensure lipidation of ATG8 and thus autophagy progression in C. reinhardtii.
© 2016 American Society of Plant Biologists. All Rights Reserved.
Figures
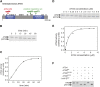


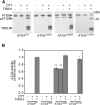

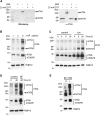




References
-
- Avila-Ospina L, Moison M, Yoshimoto K, Masclaux-Daubresse C (2014) Autophagy, plant senescence, and nutrient recycling. J Exp Bot 65: 3799–3811 - PubMed
-
- Avin-Wittenberg T, Honig A, Galili G (2012) Variations on a theme: plant autophagy in comparison to yeast and mammals. Protoplasma 249: 285–299 - PubMed
-
- Bassham DC, Laporte M, Marty F, Moriyasu Y, Ohsumi Y, Olsen LJ, Yoshimoto K (2006) Autophagy in development and stress responses of plants. Autophagy 2: 2–11 - PubMed
-
- Buchanan BB, Balmer Y (2005) Redox regulation: a broadening horizon. Annu Rev Plant Biol 56: 187–220 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

