Necroptosis-like Neuronal Cell Death Caused by Cellular Cholesterol Accumulation
- PMID: 27756839
- PMCID: PMC5122773
- DOI: 10.1074/jbc.M116.727404
Necroptosis-like Neuronal Cell Death Caused by Cellular Cholesterol Accumulation
Abstract
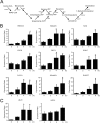
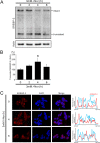
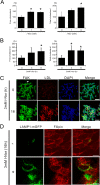
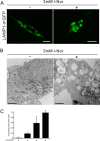
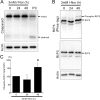
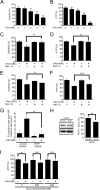
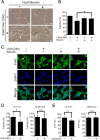
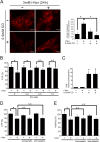
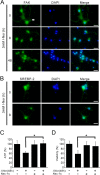
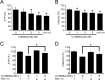
Aberrant cellular accumulation of cholesterol is associated with neuronal lysosomal storage disorders such as Niemann-Pick disease Type C (NPC). We have shown previously that l-norephedrine (l-Nor), a sympathomimetic amine, induces necrotic cell death associated with massive cytoplasmic vacuolation in SH-SY5Y human neuroblastoma cells. To reveal the molecular mechanism underling necrotic neuronal cell death caused by l-Nor, we examined alterations in the gene expression profile of cells during l-Nor exposure. DNA microarray analysis revealed that the gene levels for cholesterol transport (LDL receptor and NPC2) as well as cholesterol biosynthesis (mevalonate pathway enzymes) are increased after exposure to 3 mm l-Nor for ∼6 h. Concomitant with this observation, the master transcriptional regulator of cholesterol homeostasis, SREBP-2, is activated by l-Nor. The increase in cholesterol uptake as well as biosynthesis is not accompanied by an increase in cholesterol in the plasma membrane, but rather by aberrant accumulation in cytoplasmic compartments. We also found that cell death by l-Nor can be suppressed by nec-1s, an inhibitor of a regulated form of necrosis, necroptosis. Abrogation of SREBP-2 activation by the small molecule inhibitor betulin or by overexpression of dominant-negative SREBP-2 efficiently reduces cell death by l-Nor. The mobilization of cellular cholesterol in the presence of cyclodextrin also suppresses cell death. These results were also observed in primary culture of striatum neurons. Taken together, our results indicate that the excessive uptake as well as synthesis of cholesterol should underlie neuronal cell death by l-Nor exposure, and suggest a possible link between lysosomal cholesterol storage disorders and the regulated form of necrosis in neuronal cells.
Keywords: DNA microarray; SREBP-2; cell death; cholesterol; necroptosis; neuroblastoma; norephedrine; toxicology; transcription regulation.
© 2016 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures
Similar articles
-
Endocytosis of beta-cyclodextrins is responsible for cholesterol reduction in Niemann-Pick type C mutant cells.Proc Natl Acad Sci U S A. 2010 Mar 23;107(12):5477-82. doi: 10.1073/pnas.0914309107. Epub 2010 Mar 8. Proc Natl Acad Sci U S A. 2010. PMID: 20212119 Free PMC article.
-
Incomplete autophagy and increased cholesterol synthesis during neuronal cell death caused by a synthetic cannabinoid, CP-55,940.Neurotoxicology. 2024 Jul;103:215-221. doi: 10.1016/j.neuro.2024.06.013. Epub 2024 Jun 26. Neurotoxicology. 2024. PMID: 38942151
-
Regulation of sterol transport between membranes and NPC2.Biochemistry. 2008 Oct 21;47(42):11134-43. doi: 10.1021/bi801328u. Epub 2008 Sep 30. Biochemistry. 2008. PMID: 18823126 Free PMC article.
-
Lipids on trial: the search for the offending metabolite in Niemann-Pick type C disease.Traffic. 2010 Apr;11(4):419-28. doi: 10.1111/j.1600-0854.2010.01032.x. Epub 2010 Jan 6. Traffic. 2010. PMID: 20059748 Review.
-
Niemann-Pick Disease Type C: from molecule to clinic.Clin Exp Pharmacol Physiol. 2010 Jan;37(1):132-40. doi: 10.1111/j.1440-1681.2009.05235.x. Epub 2009 Jun 29. Clin Exp Pharmacol Physiol. 2010. PMID: 19566836 Review.
Cited by
-
Aggregation-prone A53T mutant of α-synuclein exaggerates methamphetamine neurotoxicity in SH-SY5Y cells: Protective role of cellular cholesterol.Toxicol Rep. 2022 Nov 21;9:2020-2029. doi: 10.1016/j.toxrep.2022.11.006. eCollection 2022. Toxicol Rep. 2022. PMID: 36518421 Free PMC article.
-
Induction of Lysosomal Membrane Permeabilization Is a Major Event of FTY720-Mediated Non-Apoptotic Cell Death in Human Glioma Cells.Cancers (Basel). 2020 Nov 16;12(11):3388. doi: 10.3390/cancers12113388. Cancers (Basel). 2020. PMID: 33207629 Free PMC article.
-
Cholinergic neurodegeneration and cholesterol metabolism dysregulation by constitutive p75NTR signaling in the p75exonIII-KO mice.Front Mol Neurosci. 2023 Oct 13;16:1237458. doi: 10.3389/fnmol.2023.1237458. eCollection 2023. Front Mol Neurosci. 2023. PMID: 37900943 Free PMC article.
-
Investigation of 2-Hydroxypropyl-β-Cyclodextrin Treatment in a Neuronal-Like Cell Model of Niemann-Pick Type C Using Quantitative Proteomics.J Am Soc Mass Spectrom. 2023 Apr 5;34(4):668-675. doi: 10.1021/jasms.2c00342. Epub 2023 Mar 15. J Am Soc Mass Spectrom. 2023. PMID: 36920149 Free PMC article.
-
Is autophagy an elective strategy to protect neurons from dysregulated cholesterol metabolism?Neural Regen Res. 2019 Apr;14(4):582-587. doi: 10.4103/1673-5374.247441. Neural Regen Res. 2019. PMID: 30632494 Free PMC article. Review.
References
-
- Pentel P. (1984) Toxicity of over-the-counter stimulants. JAMA 252, 1898–1903 - PubMed
-
- Bravo E. L. (1988) Phenylpropanolamine and other over-the-counter vasoactive compounds. Hypertension 11, II7–10 - PubMed
-
- Kernan W. N., Viscoli C. M., Brass L. M., Broderick J. P., Brott T., Feldmann E., Morgenstern L. B., Wilterdink J. L., and Horwitz R. I. (2000) Phenylpropanolamine and the risk of hemorrhagic stroke. N. Engl. J. Med. 343, 1826–1832 - PubMed
-
- Funakoshi T., Aki T., Unuma K., and Uemura K. (2013) Lysosome vacuolation disrupts the completion of autophagy during norephedrine exposure in SH-SY5Y human neuroblastoma cells. Brain Res. 1490, 9–22 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

