Mechanism of Sirt1 NAD+-dependent Protein Deacetylase Inhibition by Cysteine S-Nitrosation
- PMID: 27756843
- PMCID: PMC5207242
- DOI: 10.1074/jbc.M116.754655
Mechanism of Sirt1 NAD+-dependent Protein Deacetylase Inhibition by Cysteine S-Nitrosation
Abstract
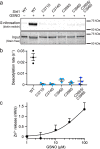
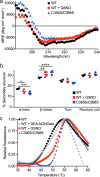
The sirtuin family of proteins catalyze the NAD+-dependent deacylation of acyl-lysine residues. Humans encode seven sirtuins (Sirt1-7), and recent studies have suggested that post-translational modification of Sirt1 by cysteine S-nitrosation correlates with increased acetylation of Sirt1 deacetylase substrates. However, the mechanism of Sirt1 inhibition by S-nitrosation was unknown. Here, we show that Sirt1 is transnitrosated and inhibited by the physiologically relevant nitrosothiol S-nitrosoglutathione. Steady-state kinetic analyses and binding assays were consistent with Sirt1 S-nitrosation inhibiting binding of both the NAD+ and acetyl-lysine substrates. Sirt1 S-nitrosation correlated with Zn2+ release from the conserved sirtuin Zn2+-tetrathiolate and a loss of α-helical structure without overall thermal destabilization of the enzyme. Molecular dynamics simulations suggested that Zn2+ loss due to Sirt1 S-nitrosation results in repositioning of the tetrathiolate subdomain away from the rest of the catalytic domain, thereby disrupting the NAD+ and acetyl-lysine-binding sites. Sirt1 S-nitrosation was reversed upon exposure to the thiol-based reducing agents, including physiologically relevant concentrations of the cellular reducing agent glutathione. Reversal of S-nitrosation resulted in full restoration of Sirt1 activity only in the presence of Zn2+, consistent with S-nitrosation of the Zn2+-tetrathiolate as the primary source of Sirt1 inhibition upon S-nitrosoglutathione treatment.
Keywords: acetylation; allosteric regulation; enzyme inactivation; inhibition mechanism; nicotinamide adenine dinucleotide (NAD); nitric oxide; nitrosation; nitrosylation; sirtuin 1 (SIRT1); zinc.
© 2016 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures





References
-
- Shinozaki S., Chang K., Sakai M., Shimizu N., Yamada M., Tanaka T., Nakazawa H., Ichinose F., Yamada Y., Ishigami A., Ito H., Ouchi Y., Starr M. E., Saito H., Shimokado K., et al. (2014) Inflammatory stimuli induce inhibitory S-nitrosylation of the deacetylase SIRT1 to increase acetylation and activation of p53 and p65. Sci. Signal. 7, ra106. - PMC - PubMed
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

