SYVN1, NEDD8, and FBXO2 Proteins Regulate ΔF508 Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Ubiquitin-mediated Proteasomal Degradation
- PMID: 27756846
- PMCID: PMC5207249
- DOI: 10.1074/jbc.M116.754283
SYVN1, NEDD8, and FBXO2 Proteins Regulate ΔF508 Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Ubiquitin-mediated Proteasomal Degradation
Abstract
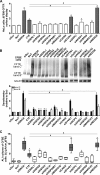
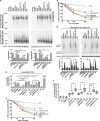
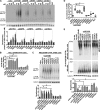
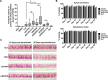
We previously reported that delivery of a microRNA-138 mimic or siRNA against SIN3A to cultured cystic fibrosis (ΔF508/ΔF508) airway epithelia partially restored ΔF508-cystic fibrosis transmembrane conductance regulator (CFTR)-mediated cAMP-stimulated Cl- conductance. We hypothesized that dissecting this microRNA-138/SIN3A-regulated gene network would identify individual proteins contributing to the rescue of ΔF508-CFTR function. Among the genes in the network, we rigorously validated candidates using functional CFTR maturation and electrolyte transport assays in polarized airway epithelia. We found that depletion of the ubiquitin ligase SYVN1, the ubiquitin/proteasome system regulator NEDD8, or the F-box protein FBXO2 partially restored ΔF508-CFTR-mediated Cl- transport in primary cultures of human cystic fibrosis airway epithelia. Moreover, knockdown of SYVN1, NEDD8, or FBXO2 in combination with corrector compound 18 further potentiated rescue of ΔF508-CFTR-mediated Cl- conductance. This study provides new knowledge of the CFTR biosynthetic pathway. It suggests that SYVN1 and FBXO2 represent two distinct multiprotein complexes that may degrade ΔF508-CFTR in airway epithelia and identifies a new role for NEDD8 in regulating ΔF508-CFTR ubiquitination.
Keywords: E3 ubiquitin ligase; airway epithelia; cystic fibrosis; endoplasmic-reticulum-associated protein degradation (ERAD); neddylation; proteostasis; ubiquitylation (ubiquitination).
© 2016 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures



Similar articles
-
Analysis of multiple gene co-expression networks to discover interactions favoring CFTR biogenesis and ΔF508-CFTR rescue.BMC Med Genomics. 2021 Oct 30;14(1):258. doi: 10.1186/s12920-021-01106-7. BMC Med Genomics. 2021. PMID: 34717611 Free PMC article.
-
Chaperone-Independent Peripheral Quality Control of CFTR by RFFL E3 Ligase.Dev Cell. 2018 Mar 26;44(6):694-708.e7. doi: 10.1016/j.devcel.2018.02.001. Epub 2018 Mar 1. Dev Cell. 2018. PMID: 29503157 Free PMC article.
-
Chaperone displacement from mutant cystic fibrosis transmembrane conductance regulator restores its function in human airway epithelia.FASEB J. 2008 Sep;22(9):3255-63. doi: 10.1096/fj.07-105338. Epub 2008 Jun 12. FASEB J. 2008. PMID: 18556464 Free PMC article.
-
Cystic fibrosis transmembrane conductance regulator degradation: cross-talk between the ubiquitylation and SUMOylation pathways.FEBS J. 2013 Sep;280(18):4430-8. doi: 10.1111/febs.12415. Epub 2013 Jul 22. FEBS J. 2013. PMID: 23809253 Free PMC article. Review.
-
Divergent signaling via SUMO modification: potential for CFTR modulation.Am J Physiol Cell Physiol. 2016 Feb 1;310(3):C175-80. doi: 10.1152/ajpcell.00124.2015. Epub 2015 Nov 18. Am J Physiol Cell Physiol. 2016. PMID: 26582473 Free PMC article. Review.
Cited by
-
The evolving role of ubiquitin modification in endoplasmic reticulum-associated degradation.Biochem J. 2017 Feb 15;474(4):445-469. doi: 10.1042/BCJ20160582. Biochem J. 2017. PMID: 28159894 Free PMC article. Review.
-
Urinary metabolomics reveals unique metabolic signatures in infants with cystic fibrosis.J Cyst Fibros. 2019 Jul;18(4):507-515. doi: 10.1016/j.jcf.2018.10.016. Epub 2018 Nov 23. J Cyst Fibros. 2019. PMID: 30477895 Free PMC article.
-
Targeting the E1 ubiquitin-activating enzyme (UBA1) improves elexacaftor/tezacaftor/ivacaftor efficacy towards F508del and rare misfolded CFTR mutants.Cell Mol Life Sci. 2022 Mar 16;79(4):192. doi: 10.1007/s00018-022-04215-3. Cell Mol Life Sci. 2022. PMID: 35292885 Free PMC article.
-
Transcriptomic and Proteostasis Networks of CFTR and the Development of Small Molecule Modulators for the Treatment of Cystic Fibrosis Lung Disease.Genes (Basel). 2020 May 13;11(5):546. doi: 10.3390/genes11050546. Genes (Basel). 2020. PMID: 32414011 Free PMC article. Review.
-
New insights into SUMOylation and NEDDylation in fibrosis.Front Pharmacol. 2024 Dec 4;15:1476699. doi: 10.3389/fphar.2024.1476699. eCollection 2024. Front Pharmacol. 2024. PMID: 39697538 Free PMC article. Review.
References
-
- Rowe S. M., Miller S., and Sorscher E. J. (2005) Cystic fibrosis. N. Engl. J. Med. 352, 1992–2001 - PubMed
-
- Anderson M. P., Gregory R. J., Thompson S., Souza D. W., Paul S., Mulligan R. C., Smith A. E., and Welsh M. J. (1991) Demonstration that CFTR is a chloride channel by alteration of its anion selectivity. Science 253, 202–205 - PubMed
-
- Anderson M. P., Rich D. P., Gregory R. J., Smith A. E., and Welsh M. J. (1991) Generation of cAMP-activated chloride currents by expression of CFTR. Science 251, 679–682 - PubMed
-
- Kerem B., Rommens J. M., Buchanan J. A., Markiewicz D., Cox T. K., Chakravarti A., Buchwald M., and Tsui L. C. (1989) Identification of the cystic fibrosis gene: genetic analysis. Science 245, 1073–1080 - PubMed
-
- Tsui L. C. (1992) Mutations and sequence variations detected in the cystic fibrosis transmembrane conductance regulator (CFTR) gene: a report from the Cystic Fibrosis Genetic Analysis Consortium. Hum. Mutat. 1, 197–203 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

