Mechanism of melanoma cells selective apoptosis induced by a photoactive NADPH analogue
- PMID: 27756874
- PMCID: PMC5347734
- DOI: 10.18632/oncotarget.12651
Mechanism of melanoma cells selective apoptosis induced by a photoactive NADPH analogue
Abstract
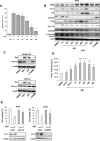
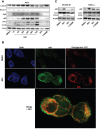
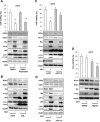
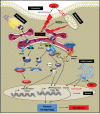
Melanoma is one of the most lethal cancers when it reaches a metastatic stage. Despite the spectacular achievements of targeted therapies (BRAF inhibitors) or immuno-therapies (anti-CTLA4 or anti-PD1), most patients with melanoma will need additional treatments. Here we used a photoactive NADPH analogue called NS1 to induce cell death by inhibition of NADPH oxidases NOX in melanoma cells, including melanoma cells isolated from patients. In contrast, healthy melanocytes growth was unaffected by NS1 treatment.NS1 established an early Endoplasmic Reticulum stress by the early release of calcium mediated by (a) calcium-dependent redox-sensitive ion channel(s). These events initiated autophagy and apoptosis in all tested melanoma cells independently of their mutational status. The autophagy promoted by NS1 was incomplete. The autophagic flux was blocked at late stage events, consistent with the accumulation of p62, and a close localization of LC3 with NS1 associated with NS1 inhibition of NOX1 in autophagosomes. This hypothesis of a specific incomplete autophagy and apoptosis driven by NS1 was comforted by the use of siRNAs and pharmacological inhibitors blocking different processes. This study highlights the potential therapeutic interest of NS1 inducing cell death by triggering a selective ER stress and incomplete autophagy in melanoma cells harbouring wt and BRAF mutation.
Keywords: ER stress; NADPH analogue; NADPH oxidase; ROS; melanoma.
Conflict of interest statement
We declare there are no competing financial interests in relation to the work described.
Figures



Similar articles
-
Regulation of NADPH-dependent Nitric Oxide and reactive oxygen species signalling in endothelial and melanoma cells by a photoactive NADPH analogue.Oncotarget. 2014 Nov 15;5(21):10650-64. doi: 10.18632/oncotarget.2525. Oncotarget. 2014. PMID: 25296975 Free PMC article.
-
Oncogenic B-RAF signaling in melanoma impairs the therapeutic advantage of autophagy inhibition.Clin Cancer Res. 2011 Apr 15;17(8):2216-26. doi: 10.1158/1078-0432.CCR-10-3003. Epub 2011 Jan 26. Clin Cancer Res. 2011. PMID: 21270111
-
Bortezomib/proteasome inhibitor triggers both apoptosis and autophagy-dependent pathways in melanoma cells.Cell Signal. 2013 Jan;25(1):308-18. doi: 10.1016/j.cellsig.2012.10.004. Epub 2012 Oct 15. Cell Signal. 2013. PMID: 23079083
-
Oncogenic BRAF, endoplasmic reticulum stress, and autophagy: Crosstalk and therapeutic targets in cutaneous melanoma.Mutat Res Rev Mutat Res. 2020 Jul-Sep;785:108321. doi: 10.1016/j.mrrev.2020.108321. Epub 2020 Jul 7. Mutat Res Rev Mutat Res. 2020. PMID: 32800272 Review.
-
Updates of reactive oxygen species in melanoma etiology and progression.Arch Biochem Biophys. 2014 Dec 1;563:51-5. doi: 10.1016/j.abb.2014.04.007. Epub 2014 Apr 26. Arch Biochem Biophys. 2014. PMID: 24780245 Free PMC article. Review.
Cited by
-
The role of mitochondria in the resistance of melanoma to PD-1 inhibitors.J Transl Med. 2023 May 23;21(1):345. doi: 10.1186/s12967-023-04200-9. J Transl Med. 2023. PMID: 37221594 Free PMC article. Review.
-
E2F1 inhibition mediates cell death of metastatic melanoma.Cell Death Dis. 2018 May 1;9(5):527. doi: 10.1038/s41419-018-0566-1. Cell Death Dis. 2018. PMID: 29743521 Free PMC article.
-
Autophagy as a targeted therapeutic approach for skin cancer: Evaluating natural and synthetic molecular interventions.Cancer Pathog Ther. 2024 Feb 1;2(4):231-245. doi: 10.1016/j.cpt.2024.01.002. eCollection 2024 Oct. Cancer Pathog Ther. 2024. PMID: 39371094 Free PMC article. Review.
-
Genetic variants in TKT and DERA in the nicotinamide adenine dinucleotide phosphate pathway predict melanoma survival.Eur J Cancer. 2020 Sep;136:84-94. doi: 10.1016/j.ejca.2020.04.049. Epub 2020 Jul 10. Eur J Cancer. 2020. PMID: 32659474 Free PMC article.
-
Dysfunctional autophagy induced by the pro-apoptotic natural compound climacostol in tumour cells.Cell Death Dis. 2018 Dec 19;10(1):10. doi: 10.1038/s41419-018-1254-x. Cell Death Dis. 2018. PMID: 30584259 Free PMC article.
References
-
- Demierre MF. Epidemiology and prevention of cutaneous melanoma. Curr Treat Options Oncol. 2006;7:181–186. - PubMed
-
- Smalley KS. Understanding melanoma signaling networks as the basis for molecular targeted therapy. J Invest Dermatol. 2010;130:28–37. - PubMed
-
- Flaherty KT, Infante JR, Daud A, Gonzalez R, Kefford RF, Sosman J, Hamid O, Schuchter L, Cebon J, Ibrahim N, Kudchadkar R, Burris HA, 3rd, Falchook G, Algazi A, Lewis K, Long GV, et al. Combined BRAF and MEK inhibition in melanoma with BRAF V600 mutations. N Engl J Med. 2012;367:1694–1703. - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

