Targeting the cancer-associated fibroblasts as a treatment in triple-negative breast cancer
- PMID: 27756881
- PMCID: PMC5341254
- DOI: 10.18632/oncotarget.12658
Targeting the cancer-associated fibroblasts as a treatment in triple-negative breast cancer
Abstract
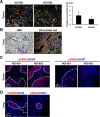
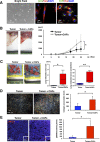
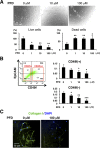
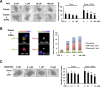
Increased collagen expression in tumors is associated with increased risk of metastasis, and triple-negative breast cancer (TNBC) has the highest propensity to develop distant metastases when there is evidence of central fibrosis. Transforming growth factor-β (TGF-β) ligands regulated by cancer-associated fibroblasts (CAFs) promote accumulation of fibrosis and cancer progression. In the present study, we have evaluated TNBC tumors with enhanced collagen to determine whether we can reduce metastasis by targeting the CAFs with Pirfenidone (PFD), an anti-fibrotic agent as well as a TGF-β antagonist. In patient-derived xenograft models, TNBC tumors exhibited accumulated collagen and activated TGF-β signaling, and developed lung metastasis. Next, primary CAFs were established from 4T1 TNBC homograft tumors, TNBC xenograft tumors and tumor specimens of breast cancer patients. CAFs promoted primary tumor growth with more fibrosis and TGF-β activation and lung metastasis in 4T1 mouse model. We then examined the effects of PFD in vitro and in vivo. We found that PFD had inhibitory effects on cell viability and collagen production of CAFs in 2D culture. Furthermore, CAFs enhanced tumor growth and PFD inhibited the tumor growth induced by CAFs by causing apoptosis in the 3D co-culture assay of 4T1 tumor cells and CAFs. In vivo, PFD alone inhibited tumor fibrosis and TGF-β signaling but did not inhibit tumor growth and lung metastasis. However, PFD inhibited tumor growth and lung metastasis synergistically in combination with doxorubicin. Thus, PFD has great potential for a novel clinically applicable TNBC therapy that targets tumor-stromal interaction.
Keywords: cancer-associated fibroblast; fibrosis; pirfenidone; transforming growth factor-β; triple-negative breast cancer.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Zerumbone suppresses the motility and tumorigenecity of triple negative breast cancer cells via the inhibition of TGF-β1 signaling pathway.Oncotarget. 2016 Jan 12;7(2):1544-58. doi: 10.18632/oncotarget.6441. Oncotarget. 2016. PMID: 26637807 Free PMC article.
-
Inhibition of iNOS as a novel effective targeted therapy against triple-negative breast cancer.Breast Cancer Res. 2015 Feb 22;17(1):25. doi: 10.1186/s13058-015-0527-x. Breast Cancer Res. 2015. PMID: 25849745 Free PMC article.
-
Stromal Fibroblasts from the Interface Zone of Triple Negative Breast Carcinomas Induced Epithelial-Mesenchymal Transition and its Inhibition by Emodin.PLoS One. 2017 Jan 6;12(1):e0164661. doi: 10.1371/journal.pone.0164661. eCollection 2017. PLoS One. 2017. PMID: 28060811 Free PMC article.
-
Targeting cancer-associated fibroblasts with pirfenidone: A novel approach for cancer therapy.Tissue Cell. 2024 Dec;91:102624. doi: 10.1016/j.tice.2024.102624. Epub 2024 Nov 19. Tissue Cell. 2024. PMID: 39581071 Review.
-
Transforming Growth Factor-β Signaling in Fibrotic Diseases and Cancer-Associated Fibroblasts.Biomolecules. 2020 Dec 12;10(12):1666. doi: 10.3390/biom10121666. Biomolecules. 2020. PMID: 33322749 Free PMC article. Review.
Cited by
-
Strategies for Efficient Targeting of Tumor Collagen for Cancer Therapy.Cancers (Basel). 2022 Sep 27;14(19):4706. doi: 10.3390/cancers14194706. Cancers (Basel). 2022. PMID: 36230627 Free PMC article. Review.
-
SPARC in cancer-associated fibroblasts is an independent poor prognostic factor in non-metastatic triple-negative breast cancer and exhibits pro-tumor activity.Int J Cancer. 2023 Mar 15;152(6):1243-1258. doi: 10.1002/ijc.34345. Epub 2022 Nov 30. Int J Cancer. 2023. PMID: 36346290 Free PMC article.
-
Cancer-associated fibroblasts in breast cancer: Challenges and opportunities.Cancer Commun (Lond). 2022 May;42(5):401-434. doi: 10.1002/cac2.12291. Epub 2022 Apr 28. Cancer Commun (Lond). 2022. PMID: 35481621 Free PMC article. Review.
-
From 3D printing to 3D bioprinting: the material properties of polymeric material and its derived bioink for achieving tissue specific architectures.Cell Tissue Bank. 2022 Sep;23(3):417-440. doi: 10.1007/s10561-021-09975-z. Epub 2022 Jan 9. Cell Tissue Bank. 2022. PMID: 35000046 Review.
-
A Human Organotypic Microfluidic Tumor Model Permits Investigation of the Interplay between Patient-Derived Fibroblasts and Breast Cancer Cells.Cancer Res. 2019 Jun 15;79(12):3139-3151. doi: 10.1158/0008-5472.CAN-18-2293. Epub 2019 Apr 16. Cancer Res. 2019. PMID: 30992322 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

