Bevacizumab and wound-healing complications: a systematic review and meta-analysis of randomized controlled trials
- PMID: 27756883
- PMCID: PMC5347706
- DOI: 10.18632/oncotarget.12666
Bevacizumab and wound-healing complications: a systematic review and meta-analysis of randomized controlled trials
Abstract
A meta-analysis was conducted to estimate the risk of wound-healing complications in patients who treated with neoadjuvant-adjuvant bevacizumab in various oncological indications. We searched PUBMED, EMBASE and the Cochrane Library through June 2016 to identify randomized controlled trials of bevacizumab and wound-healing complications. Seven RCTs studies involving 5,147 participants were included in the analysis. Compared with routine therapy, bevacizumab increased the incidence of wound-healing complications for various cancers. The pooled estimate of odds ratio (OR) was 2.32, and the 95 % confidence intervals (CI) was 1.43 to 3.75. (P < 0.001). Subgroup analyses revealed the similar result in colon carcinoma patients. In conclusion, bevacizumab increases the incidence of wound-healing complications for cancers especially for colon neoplasms patients. However, the adverse effect is not appeared in breast cancer, metastatic renal cell carcinoma, non-small-cell lung cancer and gastro-oesophageal adenocarcinoma. Due to the findings relying chiefly on data from single or two studies, hence, further research is required to assess the wound-healing complications risk of bevacizumab in each oncological indication.
Keywords: bevacizumab; meta-analysis; systematic review; wound-healing complications.
Conflict of interest statement
The authors have reported no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Figures
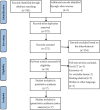
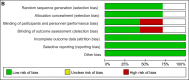




References
-
- Sharma K, Marcus JR. Bevacizumab and wound-healing complications: mechanisms of action, clinical evidence, and management recommendations for the plastic surgeon. Ann Plast Surg. 2013;71:434–440. - PubMed
-
- FDA Commissioner Removes Breast Cancer Indication from Avastin Label 2011 Nov 18; http://wwwfdagov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPati...
-
- Mansour J, Fields B, Macomson S, Rixe O. Significant anti-tumor effect of bevacizumab in treatment of pineal gland glioblastoma multiforme. Target Oncol. 2014;9:395–398. - PubMed
-
- Ellis LM. Mechanisms of action of bevacizumab as a component of therapy for metastatic colorectal cancer. Semin Oncol. 2006;33:S1–7. - PubMed
-
- Nakahara T, Norberg SM, Shalinsky DR, Hu-Lowe DD, McDonald DM. Effect of inhibition of vascular endothelial growth factor signaling on distribution of extravasated antibodies in tumors. Cancer Res. 2006;66:1434–1445. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

