A rare schizophrenia risk variant of CACNA1I disrupts CaV3.3 channel activity
- PMID: 27756899
- PMCID: PMC5069464
- DOI: 10.1038/srep34233
A rare schizophrenia risk variant of CACNA1I disrupts CaV3.3 channel activity
Abstract
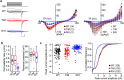
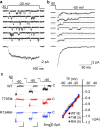
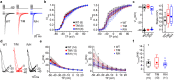
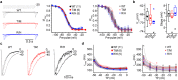
CACNA1I is a candidate schizophrenia risk gene. It encodes the pore-forming human CaV3.3 α1 subunit, a subtype of voltage-gated calcium channel that contributes to T-type currents. Recently, two de novo missense variations, T797M and R1346H, of hCaV3.3 were identified in individuals with schizophrenia. Here we show that R1346H, but not T797M, is associated with lower hCaV3.3 protein levels, reduced glycosylation, and lower membrane surface levels of hCaV3.3 when expressed in human cell lines compared to wild-type. Consistent with our biochemical analyses, whole-cell hCaV3.3 currents in cells expressing the R1346H variant were ~50% of those in cells expressing WT hCaV3.3, and neither R1346H nor T797M altered channel biophysical properties. Employing the NEURON simulation environment, we found that reducing hCaV3.3 current densities by 22% or more eliminates rebound bursting in model thalamic reticular nucleus (TRN) neurons. Our analyses suggest that a single copy of Chr22: 39665939G > A CACNA1I has the capacity to disrupt CaV3.3 channel-dependent functions, including rebound bursting in TRN neurons, with potential implications for schizophrenia pathophysiology.
Figures



Similar articles
-
Analysing an allelic series of rare missense variants of CACNA1I in a Swedish schizophrenia cohort.Brain. 2022 Jun 3;145(5):1839-1853. doi: 10.1093/brain/awab443. Brain. 2022. PMID: 34919654 Free PMC article.
-
Effects of a patient-derived de novo coding alteration of CACNA1I in mice connect a schizophrenia risk gene with sleep spindle deficits.Transl Psychiatry. 2020 Jan 23;10(1):29. doi: 10.1038/s41398-020-0685-1. Transl Psychiatry. 2020. PMID: 32066662 Free PMC article.
-
CACNA1I gain-of-function mutations differentially affect channel gating and cause neurodevelopmental disorders.Brain. 2021 Aug 17;144(7):2092-2106. doi: 10.1093/brain/awab101. Brain. 2021. PMID: 33704440 Free PMC article.
-
Neuronal Cav3 channelopathies: recent progress and perspectives.Pflugers Arch. 2020 Jul;472(7):831-844. doi: 10.1007/s00424-020-02429-7. Epub 2020 Jul 7. Pflugers Arch. 2020. PMID: 32638069 Free PMC article. Review.
-
[Cognitive Function and Calcium. Cognitive improvement through T type calcium channel stimulation].Clin Calcium. 2015 Feb;25(2):247-54. Clin Calcium. 2015. PMID: 25634050 Review. Japanese.
Cited by
-
Epigenome-wide DNA methylation in leukocytes and toenail metals: The normative aging study.Environ Res. 2023 Jan 15;217:114797. doi: 10.1016/j.envres.2022.114797. Epub 2022 Nov 12. Environ Res. 2023. PMID: 36379232 Free PMC article.
-
Genetic risk between the CACNA1I gene and schizophrenia in Chinese Uygur population.Hereditas. 2017 Jul 17;155:5. doi: 10.1186/s41065-017-0037-1. eCollection 2018. Hereditas. 2017. PMID: 28725167 Free PMC article.
-
The thalamic reticular nucleus in schizophrenia and bipolar disorder: role of parvalbumin-expressing neuron networks and oxidative stress.Mol Psychiatry. 2018 Oct;23(10):2057-2065. doi: 10.1038/mp.2017.230. Epub 2017 Nov 28. Mol Psychiatry. 2018. PMID: 29180672 Free PMC article.
-
Genetic correlations among psychiatric and immune-related phenotypes based on genome-wide association data.Am J Med Genet B Neuropsychiatr Genet. 2018 Oct;177(7):641-657. doi: 10.1002/ajmg.b.32652. Epub 2018 Oct 16. Am J Med Genet B Neuropsychiatr Genet. 2018. PMID: 30325587 Free PMC article.
-
Identification of ultra-rare disruptive variants in voltage-gated calcium channel-encoding genes in Japanese samples of schizophrenia and autism spectrum disorder.Transl Psychiatry. 2022 Feb 26;12(1):84. doi: 10.1038/s41398-022-01851-y. Transl Psychiatry. 2022. PMID: 35220405 Free PMC article.
References
-
- Perez-Reyes E. Molecular physiology of low-voltage-activated t-type calcium channels. Physiol Rev 83, 117–161 (2003). - PubMed
-
- Houser C. R., Vaughn J. E., Barber R. P. & Roberts E. GABA neurons are the major cell type of the nucleus reticularis thalami. Brain Res 200, 341–354 (1980). - PubMed
-
- Liu X. B., Murray K. D. & Jones E. G. Low-threshold calcium channel subunit Ca(v) 3.3 is specifically localized in GABAergic neurons of rodent thalamus and cerebral cortex. J Comp Neurol 519, 1181–1195 (2011). - PubMed
-
- Steriade M. Grouping of brain rhythms in corticothalamic systems. Neuroscience 137, 1087–1106 (2006). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

