Chronic stress prior to pregnancy potentiated long-lasting postpartum depressive-like behavior, regulated by Akt-mTOR signaling in the hippocampus
- PMID: 27756905
- PMCID: PMC5069466
- DOI: 10.1038/srep35042
Chronic stress prior to pregnancy potentiated long-lasting postpartum depressive-like behavior, regulated by Akt-mTOR signaling in the hippocampus
Abstract
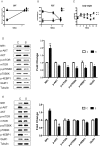
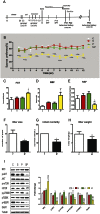
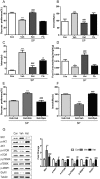
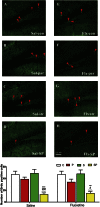
Postpartum depression (PPD) affects over 10% of new mothers and adversely impacts the health of offspring. One of the greatest risk factors for PPD is prepregnancy stress but the underlying biological mechanism is unknown. Here we constructed an animal model which recapitulated prepregnancy stress induced PPD and tested the role of Akt-mTOR signaling in the hippocampus. Female virgin Balb/c mice received chronic restraint stress, followed by co-housing with a normal male mouse. We found that the chronic stress led to a transient depressive-like condition that disappeared within two weeks. However, prepregnantly stressed females developed long-term postpartum depressive-like (PPD-like) symptoms as indicated by deficient performance in tests of sucrose preference, forced swim, and novelty-suppressed feeding. Chronic stress induced transient decrease in Akt-mTOR signaling and altered expressions of glutamate receptor subunits NR1 and GluR1, in contrast to long-term deficits in Akt-mTOR signaling, GluR1/NR1 ratio, and hippocampal neurogenesis in PPD-like mice. Acute ketamine improved the molecular signaling abnormality, and reversed the behavioral deficits in PPD-like mice in a rapid and persistent manner, in contrast to ineffectiveness by chronic fluoxetine treatment. Taken together, we find that chronic prepregnancy stress potentiates a long-term PPD, in which Akt-mTOR signaling may play a crucial role.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
Similar articles
-
Transgenerational impairment of hippocampal Akt-mTOR signaling and behavioral deficits in the offspring of mice that experience postpartum depression-like illness.Prog Neuropsychopharmacol Biol Psychiatry. 2017 Feb 6;73:11-18. doi: 10.1016/j.pnpbp.2016.09.008. Epub 2016 Sep 28. Prog Neuropsychopharmacol Biol Psychiatry. 2017. PMID: 27693392
-
Involvement of normalized NMDA receptor and mTOR-related signaling in rapid antidepressant effects of Yueju and ketamine on chronically stressed mice.Sci Rep. 2015 Aug 28;5:13573. doi: 10.1038/srep13573. Sci Rep. 2015. PMID: 26315757 Free PMC article.
-
Antidepressant effects of esketamine via the BDNF/AKT/mTOR pathway in mice with postpartum depression and their offspring.Prog Neuropsychopharmacol Biol Psychiatry. 2024 Jun 8;132:110992. doi: 10.1016/j.pnpbp.2024.110992. Epub 2024 Mar 13. Prog Neuropsychopharmacol Biol Psychiatry. 2024. PMID: 38484929
-
PI3K/AKT/mTOR signaling-mediated neuropeptide VGF in the hippocampus of mice is involved in the rapid onset antidepressant-like effects of GLYX-13.Int J Neuropsychopharmacol. 2014 Dec 25;18(5):pyu110. doi: 10.1093/ijnp/pyu110. Int J Neuropsychopharmacol. 2014. PMID: 25542689 Free PMC article.
-
Ketamine as a prophylactic resilience-enhancing agent.Front Psychiatry. 2022 Jul 28;13:833259. doi: 10.3389/fpsyt.2022.833259. eCollection 2022. Front Psychiatry. 2022. PMID: 35966469 Free PMC article. Review.
Cited by
-
The Role of the FOXO1/β2-AR/p-NF-κB p65 Pathway in the Development of Endometrial Stromal Cells in Pregnant Mice under Restraint Stress.Int J Mol Sci. 2021 Feb 2;22(3):1478. doi: 10.3390/ijms22031478. Int J Mol Sci. 2021. PMID: 33540675 Free PMC article.
-
Loss of Glutamate Decarboxylase 67 in Somatostatin-Expressing Neurons Leads to Anxiety-Like Behavior and Alteration in the Akt/GSK3β Signaling Pathway.Front Behav Neurosci. 2019 Jun 18;13:131. doi: 10.3389/fnbeh.2019.00131. eCollection 2019. Front Behav Neurosci. 2019. PMID: 31275123 Free PMC article.
-
Regulation of Cortico-Thalamic JNK1/2 and ERK1/2 MAPKs and Apoptosis-Related Signaling Pathways in PDYN Gene-Deficient Mice Following Acute and Chronic Mild Stress.Int J Mol Sci. 2023 Jan 24;24(3):2303. doi: 10.3390/ijms24032303. Int J Mol Sci. 2023. PMID: 36768626 Free PMC article.
-
Identification of 14-3-3 epsilon as a regulator of the neural apoptotic pathway for chronic-stress-induced depression.iScience. 2021 Jan 8;24(2):102043. doi: 10.1016/j.isci.2021.102043. eCollection 2021 Feb 19. iScience. 2021. PMID: 33537655 Free PMC article.
-
Increased depression-related behavior during the postpartum period in inbred BALB/c and C57BL/6 strains.Mol Brain. 2019 Aug 9;12(1):70. doi: 10.1186/s13041-019-0490-z. Mol Brain. 2019. PMID: 31399102 Free PMC article.
References
-
- Friedman S. H. & Resnick P. J. Postpartum depression: an update. Women’s Health 5, 287–295 (2009). - PubMed
-
- Josefsson A., Berg G., Nordin C. & Sydsjö G. Prevalence of depressive symptoms in late pregnancy and postpartum. Acta Obstetricia et Gynecologica Scandinavica 80, 251–255 (2001). - PubMed
-
- Atkinson L. et al. Attachment security: A meta-analysis of maternal mental health correlates. Clinical Psychology Review 20, 1019–1040 (2000). - PubMed
-
- Lovejoy M. C., Graczyk P. A., O’Hare E. & Neuman G. Maternal depression and parenting behavior: A meta-analytic review. Clinical Psychology Review 20, 561–592 (2000). - PubMed
-
- Beck C. T. Predictors of Postpartum Depression: An Update. Nursing Research 50 (2001). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

