A nanoporous surface is essential for glomerular podocyte differentiation in three-dimensional culture
- PMID: 27757030
- PMCID: PMC5053378
- DOI: 10.2147/IJN.S110201
A nanoporous surface is essential for glomerular podocyte differentiation in three-dimensional culture
Abstract
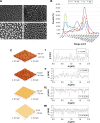
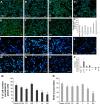
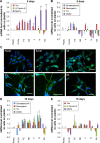
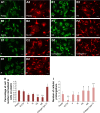
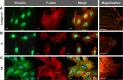
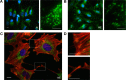
Although it is well recognized that cell-matrix interactions are based on both molecular and geometrical characteristics, the relationship between specific cell types and the three-dimensional morphology of the surface to which they are attached is poorly understood. This is particularly true for glomerular podocytes - the gatekeepers of glomerular filtration - which completely enwrap the glomerular basement membrane with their primary and secondary ramifications. Nanotechnologies produce biocompatible materials which offer the possibility to build substrates which differ only by topology in order to mimic the spatial organization of diverse basement membranes. With this in mind, we produced and utilized rough and porous surfaces obtained from silicon to analyze the behavior of two diverse ramified cells: glomerular podocytes and a neuronal cell line used as a control. Proper differentiation and development of ramifications of both cell types was largely influenced by topographical characteristics. Confirming previous data, the neuronal cell line acquired features of maturation on rough nanosurfaces. In contrast, podocytes developed and matured preferentially on nanoporous surfaces provided with grooves, as shown by the organization of the actin cytoskeleton stress fibers and the proper development of vinculin-positive focal adhesions. On the basis of these findings, we suggest that in vitro studies regarding podocyte attachment to the glomerular basement membrane should take into account the geometrical properties of the surface on which the tests are conducted because physiological cellular activity depends on the three-dimensional microenvironment.
Keywords: adhesion; basement membrane; differentiation; nanotechnology; neurons; podocytes.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures





References
-
- Lim JY, Dreiss AD, Zhou Z, et al. The regulation of integrin-mediated osteoblast focal adhesion and focal adhesion kinase expression by nanoscale topography. Biomaterials. 2007;28(10):1787–1797. - PubMed
-
- Migliorini E, Grenci G, Ban J, et al. Acceleration of neuronal precursors differentiation induced by substrate nanotopography. Biotechnol Bioeng. 2011;108(11):2736–2746. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

