What are we paying for? A cost-effectiveness analysis of patented denosumab and generic alendronate for postmenopausal osteoporotic women in Australia
- PMID: 27757069
- PMCID: PMC5064794
- DOI: 10.1186/s12962-016-0060-5
What are we paying for? A cost-effectiveness analysis of patented denosumab and generic alendronate for postmenopausal osteoporotic women in Australia
Abstract
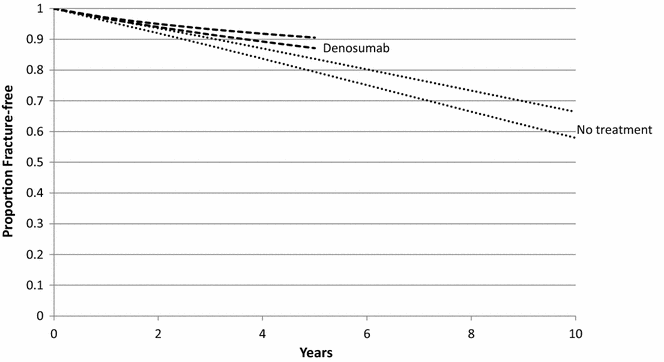
Objective: Zoledronic acid and denosumab were funded by the Australian government for the management of osteoporosis at an equivalent price to alendronate. The price of alendronate has declined by around 65 %, but the price of the other two therapies has remained stable. Using data published since the listing, this paper reports current estimates of the value of denosumab compared to alendronate from an Australian health system perspective.
Methods: A cohort-based state transition model was developed that predicted changes in bone mineral density (BMD), and calibrated fracture probabilities as a function of BMD, age and previous fracture to estimate differences in costs and QALYs gained over a 10-year time horizon.
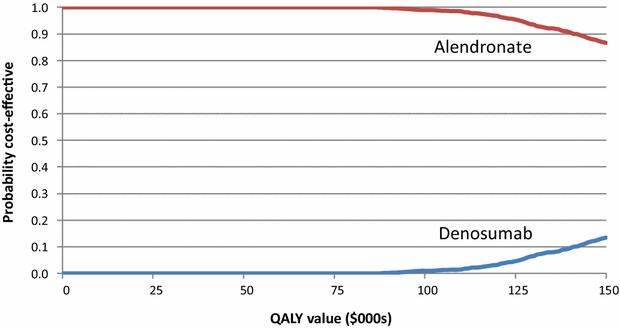
Results: The base-case incremental cost per QALY gained for denosumab versus alendronate was $246,749. There is a near zero probability that denosumab is cost-effective at a threshold value of $100,000 per QALY gained. If the price of denosumab was reduced by 50 %, the incremental cost per QALY gained falls to $50,068.
Discussion: Current Australian legislation precludes price reviews when comparator therapies come off patent. The presented analysis illustrates a review process, incorporating clinical data collected since the original submission to inform a price at which denosumab would provide value for money.
Keywords: Calibration; Cost-effectiveness; Generic; Osteoporosis; Patent; Pharmaceutical pricing.
Figures
Similar articles
-
Cost effectiveness of denosumab versus oral bisphosphonates for postmenopausal osteoporosis in the US.Appl Health Econ Health Policy. 2013 Oct;11(5):485-97. doi: 10.1007/s40258-013-0047-8. Appl Health Econ Health Policy. 2013. PMID: 23868102
-
Cost effectiveness of denosumab compared with oral bisphosphonates in the treatment of post-menopausal osteoporotic women in Belgium.Pharmacoeconomics. 2011 Oct;29(10):895-911. doi: 10.2165/11539980-000000000-00000. Pharmacoeconomics. 2011. PMID: 21692551
-
Cost-effectiveness of zoledronic acid compared with sequential denosumab/alendronate for older osteoporotic women in Japan.Arch Osteoporos. 2021 Jul 15;16(1):113. doi: 10.1007/s11657-021-00956-z. Arch Osteoporos. 2021. PMID: 34264429 Free PMC article.
-
CLINICAL EVALUATION OF COST EFFICACY OF DRUGS FOR TREATMENT OF OSTEOPOROSIS: A META-ANALYSIS.Endocr Pract. 2017 Jul;23(7):841-856. doi: 10.4158/EP161678.RA. Epub 2017 Apr 27. Endocr Pract. 2017. PMID: 28448754 Review.
-
Multi-gene Pharmacogenomic Testing That Includes Decision-Support Tools to Guide Medication Selection for Major Depression: A Health Technology Assessment.Ont Health Technol Assess Ser. 2021 Aug 12;21(13):1-214. eCollection 2021. Ont Health Technol Assess Ser. 2021. PMID: 34484487 Free PMC article.
Cited by
-
An Updated Systematic Review of Cost-Effectiveness Analyses of Drugs for Osteoporosis.Pharmacoeconomics. 2021 Feb;39(2):181-209. doi: 10.1007/s40273-020-00965-9. Epub 2020 Oct 7. Pharmacoeconomics. 2021. PMID: 33026634 Free PMC article.
-
Cost-Effectiveness of Pharmacological Treatments for Osteoporosis Consistent with the Revised Economic Evaluation Guidelines for Canada.MDM Policy Pract. 2019 Jan 30;4(1):2381468318818843. doi: 10.1177/2381468318818843. eCollection 2019 Jan-Jun. MDM Policy Pract. 2019. PMID: 30729168 Free PMC article.
-
Equivalence trial of proposed denosumab biosimilar GP2411 and reference denosumab in postmenopausal osteoporosis: the ROSALIA study.J Bone Miner Res. 2024 Apr 19;39(3):202-210. doi: 10.1093/jbmr/zjae016. J Bone Miner Res. 2024. PMID: 38477751 Free PMC article. Clinical Trial.
-
Systematic evidence of health economic evaluation of drugs for postmenopausal osteoporosis: A quality appraisal.Osteoporos Sarcopenia. 2020 Jun;6(2):39-52. doi: 10.1016/j.afos.2020.05.006. Epub 2020 Jun 23. Osteoporos Sarcopenia. 2020. PMID: 32715093 Free PMC article. Review.
-
The cost-effectiveness of osteoporosis medications for preventing periprosthetic fractures following femoral neck fracture indicated hip arthroplasty: a break-even analysis.Osteoporos Int. 2024 Jul;35(7):1223-1229. doi: 10.1007/s00198-024-07085-6. Epub 2024 Apr 15. Osteoporos Int. 2024. PMID: 38619605
References
-
- Watt JJ, Abimanyl-Ochom J, Sanders KM. Osteoporosis costing all Australians. A new burden of disease analysis-2012–2022, Osteoporosis Australia 2012, ISBN 978-0-9923698-1-1.
-
- Karnon J, Edney L, Sorich M. Costs of paying higher prices for equivalent effects on the pharmaceutical benefits scheme. Aust Health Rev. http://dx.doi.org/10.1071/AH15122. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources