Influence of Biphasic Stimulation on Olfactory Ensheathing Cells for Neuroprosthetic Devices
- PMID: 27757072
- PMCID: PMC5048075
- DOI: 10.3389/fnins.2016.00432
Influence of Biphasic Stimulation on Olfactory Ensheathing Cells for Neuroprosthetic Devices
Abstract
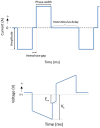
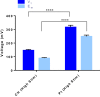
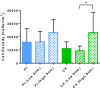
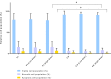
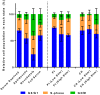
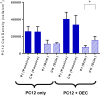
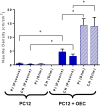
The recent success of olfactory ensheathing cell (OEC) assisted regeneration of injured spinal cord has seen a rising interest in the use of these cells in tissue-engineered systems. Previously shown to support neural cell growth through glial scar tissue, OECs have the potential to assist neural network formation in living electrode systems to produce superior neuroprosthetic electrode surfaces. The following study sought to understand the influence of biphasic electrical stimulation (ES), inherent to bionic devices, on cell survival and function, with respect to conventional metallic and developmental conductive hydrogel (CH) coated electrodes. The CH utilized in this study was a biosynthetic hydrogel consisting of methacrylated poly(vinyl-alcohol) (PVA), heparin and gelatin through which poly(3,4-ethylenedioxythiophene) (PEDOT) was electropolymerised. OECs cultured on Pt and CH surfaces were subjected to biphasic ES. Image-based cytometry yielded little significant difference between the viability and cell cycle of OECs cultured on the stimulated and passive samples. The significantly lower voltages measured across the CH electrodes (147 ± 3 mV) compared to the Pt (317 ± 5 mV), had shown to influence a higher percentage of viable cells on CH (91-93%) compared to Pt (78-81%). To determine the functionality of these cells following electrical stimulation, OECs co-cultured with PC12 cells were found to support neural cell differentiation (an indirect measure of neurotrophic factor production) following ES.
Keywords: PEDOT; electrical stimulation of nervous system; living electrodes; neural interfaces; olfactory ensheathing cells.
Figures



Similar articles
-
Biofunctionalization of conductive hydrogel coatings to support olfactory ensheathing cells at implantable electrode interfaces.J Biomed Mater Res B Appl Biomater. 2016 May;104(4):712-22. doi: 10.1002/jbm.b.33497. Epub 2015 Aug 6. J Biomed Mater Res B Appl Biomater. 2016. PMID: 26248597
-
BDNF production by olfactory ensheathing cells contributes to axonal regeneration of cultured adult CNS neurons.Neurochem Int. 2007 Feb;50(3):491-8. doi: 10.1016/j.neuint.2006.10.004. Epub 2006 Dec 8. Neurochem Int. 2007. PMID: 17157963
-
Transcription factor Runx1 inhibits proliferation and promotes developmental maturation in a selected population of inner olfactory nerve layer olfactory ensheathing cells.Gene. 2014 May 1;540(2):191-200. doi: 10.1016/j.gene.2014.02.038. Epub 2014 Feb 26. Gene. 2014. PMID: 24582971
-
Understanding the neural repair-promoting properties of olfactory ensheathing cells.Exp Neurol. 2014 Nov;261:594-609. doi: 10.1016/j.expneurol.2014.05.007. Epub 2014 May 17. Exp Neurol. 2014. PMID: 24842489 Review.
-
Interaction of olfactory ensheathing cells with other cell types in vitro and after transplantation: glial scars and inflammation.Exp Neurol. 2011 May;229(1):46-53. doi: 10.1016/j.expneurol.2010.08.012. Epub 2010 Aug 14. Exp Neurol. 2011. PMID: 20713050 Review.
Cited by
-
Functional and Biomimetic Materials for Engineering of the Three-Dimensional Cell Microenvironment.Chem Rev. 2017 Oct 25;117(20):12764-12850. doi: 10.1021/acs.chemrev.7b00094. Epub 2017 Oct 9. Chem Rev. 2017. PMID: 28991456 Free PMC article. Review.
-
Conductive Hydrogel Electrodes for Delivery of Long-Term High Frequency Pulses.Front Neurosci. 2018 Jan 11;11:748. doi: 10.3389/fnins.2017.00748. eCollection 2017. Front Neurosci. 2018. PMID: 29375292 Free PMC article.
References
-
- Ansselin A. D., Fink T., Davey D. F. (1997). Peripheral nerve regeneration through nerve guides seeded with adult Schwann cells. Neuropathol. Appl. Neurobiol. 23, 387–398. - PubMed
-
- Chan R. T. H., Garvey C. J., Marçal H., Russell R. A., Holden P. J., Foster L. J. R. (2011). Manipulation of polyhydroxybutyrate properties through blending with ethyl-cellulose for a composite biomaterial. Int. J. Polym. Sci. 2011:651549 10.1155/2011/651549 - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

