Targeting myeloid-derived suppressor cells using a novel adenosine monophosphate-activated protein kinase (AMPK) activator
- PMID: 27757311
- PMCID: PMC5048767
- DOI: 10.1080/2162402X.2016.1214787
Targeting myeloid-derived suppressor cells using a novel adenosine monophosphate-activated protein kinase (AMPK) activator
Abstract
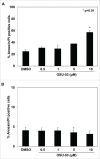
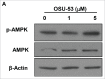
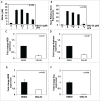
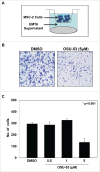
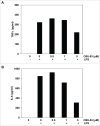
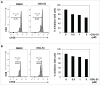
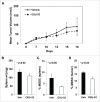
Myeloid-derived suppressor cells (MDSC) are a heterogeneous population of early myeloid cells that accumulate in the blood and tumors of patients with cancer. MDSC play a critical role during tumor evasion and promote immune suppression through variety of mechanisms, such as the generation of reactive oxygen and nitrogen species (ROS and RNS) and cytokines. AMPactivated protein kinase (AMPK) is an evolutionarily conserved serine/threonine kinase that regulates energy homeostasis and metabolic stress. However, the role of AMPK in the regulation of MDSC function remains largely unexplored. This study was designed to investigate whether treatment of MDSC with OSU-53, a PPAR-inactive derivative that stimulates AMPK kinase, can modulate MDSC function. Our results demonstrate that OSU-53 treatment increases the phosphorylation of AMPK, significantly reduces nitric oxide production, inhibits MDSC migration, and reduces the levels of IL-6 in murine MDSC cell line (MSC2 cells). OSU53 treatment mitigated the immune suppressive functions of murine MDSC, promoting T-cell proliferation. Although OSU-53 had a modest effect on tumor growth in mice inoculated with EMT-6 cells, importantly, administration of OSU53 significantly (p < 0.05) reduced the levels of MDSC in the spleens and tumors. Furthermore, mouse MDSC from EMT-6 tumor-bearing mice and human MDSC isolated from melanoma patients treated with OSU-53 showed a significant reduction in the expression of immune suppressive genes iNOS and arginase. In summary, these results demonstrate a novel role of AMPK in the regulation of MDSC functions and provide a rationale of combining OSU-53 with immune checkpoint inhibitors to augment their response in cancer patients.
Keywords: AMPK; Immunotherapy; MDSC; OSU-53; iNOS.
Figures
References
-
- Bissell MJ, Hines WC. Why don't we get more cancer? A proposed role of the microenvironment in restraining cancer progression. Nat Med 2011; 17:320-9; PMID:21383745; http://dx.doi.org/10.1038/nm.2328 - DOI - PMC - PubMed
-
- Lindau D, Gielen P, Kroesen M, Wesseling P, Adema GJ. The immunosuppressive tumour network: myeloid-derived suppressor cells, regulatory T cells and natural killer T cells. Immunology 2013; 138:105-15; PMID:23216602; http://dx.doi.org/10.1111/imm.12036 - DOI - PMC - PubMed
-
- Solito S, Marigo I, Pinton L, Damuzzo V, Mandruzzato S, Bronte V. Myeloid-derived suppressor cell heterogeneity in human cancers. Ann N Y Acad Sci 2014; 1319:47-65; PMID:24965257; http://dx.doi.org/10.1111/nyas.12469 - DOI - PubMed
-
- Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol 2009; 9:162-74; PMID:19197294; http://dx.doi.org/10.1038/nri2506 - DOI - PMC - PubMed
-
- Trikha P, Carson WE. Signaling pathways involved in MDSC regulation. Biochim Biophys Acta 2014. Aug; 1846(1):55-65; PMID:24727385; http://dx.doi.org/10.1016/j.bbcan.2014.04.003 - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
