Pathological TDP-43 changes in Betz cells differ from those in bulbar and spinal α-motoneurons in sporadic amyotrophic lateral sclerosis
- PMID: 27757524
- PMCID: PMC5209403
- DOI: 10.1007/s00401-016-1633-2
Pathological TDP-43 changes in Betz cells differ from those in bulbar and spinal α-motoneurons in sporadic amyotrophic lateral sclerosis
Abstract
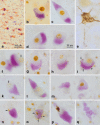
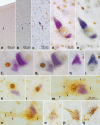
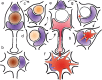
Two nerve cells types, Betz cells in layer Vb of the primary motor neocortex and α-motoneurons of the lower brainstem and spinal cord, become involved at the beginning of the pathological cascade underlying sporadic amyotrophic lateral sclerosis (sALS). In both neuronal types, the cell nuclei forfeit their normal (non-phosphorylated) expression of the 43-kDa transactive response DNA-binding protein (TDP-43). Here, we present initial evidence that in α-motoneurons the loss of normal nuclear TDP-43 expression is followed by the formation of phosphorylated TDP-43 aggregates (pTDP-43) within the cytoplasm, whereas in Betz cells, by contrast, the loss of normal nuclear TDP-43 expression remains mostly unaccompanied by the development of cytoplasmic aggregations. We discuss some implications of this phenomenon of nuclear clearing in the absence of cytoplasmic inclusions, namely, abnormal but soluble (and, thus, probably toxic) cytoplasmic TDP-43 could enter the axoplasm of Betz cells, and following its transmission to the corresponding α-motoneurons in the lower brainstem and spinal cord, possibly contribute in recipient neurons to the dysregulation of the normal nuclear protein. Because the cellular mechanisms that possibly inhibit the aggregation of TDP-43 in the cytoplasm of involved Betz cells are unknown, insight into such mechanisms could disclose a pathway by which the development of aggregates in this cell population could be accelerated, thereby opening an avenue for a causally based therapy.
Keywords: Amyotrophic lateral sclerosis; Betz cells; Motor neuron disease; Primary motor cortex; TAR DNA-binding protein; TDP-43; Therapeutics; Transsynaptic spreading; α-Motoneurons.
Conflict of interest statement
The authors have no current or potential conflicts of interest.
Figures
References
-
- Betz W. Anatomischer Nachweis zweier Gehirncentra. Centralblatt für die medizinischen Wissenschaften. 1874;12(578–580):595–599.
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

