Cementogenic genes in human periodontal ligament stem cells are downregulated in response to osteogenic stimulation while upregulated by vitamin C treatment
- PMID: 27757536
- PMCID: PMC5366101
- DOI: 10.1007/s00441-016-2513-8
Cementogenic genes in human periodontal ligament stem cells are downregulated in response to osteogenic stimulation while upregulated by vitamin C treatment
Erratum in
-
Erratum to: Cementogenic genes in human periodontal ligament stem cells are downregulated in response to osteogenic stimulation while upregulated by vitamin C treatment.Cell Tissue Res. 2017 Apr;368(1):227. doi: 10.1007/s00441-016-2550-3. Cell Tissue Res. 2017. PMID: 27981381 No abstract available.
Abstract
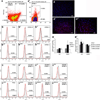
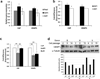
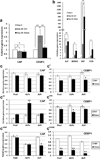
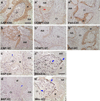
Regeneration of periodontal tissues, particularly cementum, is key to regaining periodontal attachment and health. Human periodontal ligament stem cells (hPDLSCs) have been shown to be a good cell source to regenerate periodontal tissues. However, their subpopulations and the differentiation induction in relation to cementogenic lineages is unclear. Thus, we aim to examine the expression of cementum-associated genes in PDLSC subpopulations and determine the effect of broadly used osteogenic stimulus or vitamin C (VC) on the expression of cementogenic and osteogenic genes in PDLSCs. Our real-time quantitative polymerase chain reaction (qPCR) analysis showed that cementogenic marker cementum attachment protein (CAP) expressed only slightly higher in STRO-1+/CD146+, STRO-1-/CD146+ and STRO-1-/CD146- subpopulations than in the original cell pool, while cementum protein 1 (CEMP1) expression in these subpopulations was not different from the original pool. Notably, under the stimulation with osteogenic differentiation medium, CAP and CEMP1 were downregulated while osteogenic markers bone sialoprotein (BSP) and osteocalcin (OCN) were upregulated. Both CAP and CEMP1 were upregulated by VC treatment. Transplantation of VC-treated PDLSCs into immunocompromised mice resulted in forming significantly more ectopic cementum- and bone-like mineral tissues in vivo. Immunohistochemical analysis of the ectopic growth showed that CAP and CEMP1 were mainly expressed in the mineral tissue and in some cells of the fibrous tissues. We conclude that osteogenic stimulation is not inductive but appears to be inhibitory of cementogenic pathways, whereas VC induces cementogenic lineage commitment by PDLSCs and may be a useful stimulus for cementogenesis in periodontal regeneration.
Keywords: Cementum attachment protein; Cementum protein 1; Osteogenic induction; Periodontal ligament stem cells; Vitamin C.
Figures


References
-
- Akizuki T, Oda S, Komaki M, Tsuchioka H, Kawakatsu N, Kikuchi A, Yamato M, Okano T, Ishikawa I. Application of periodontal ligament cell sheet for periodontal regeneration: a pilot study in beagle dogs. J Periodontal Res. 2005;40:245–251. - PubMed
-
- Alvarez-Pérez MA, Narayanan S, Zeichner-David M, Rodríguez Carmona B, Arzate H. Molecular cloning, expression and immunolocalization of a novel human cementum-derived protein (CP-23) Bone. 2006;38:409–419. - PubMed
-
- Arzate H, Olson SW, Page RC, Gown AM, Narayanan AS. Production of a monoclonal antibody to an attachment protein derived from human cementum. FASEB J. 1992;6:2990–2995. - PubMed
-
- BarKana I, Narayanan AS, Grosskop A, Savion N, Pitaru S. Cementum attachment protein enriches putative cementoblastic populations on root surfaces in vitro. J Dent Res. 2000;79:1482–1488. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

