Regulation of gap junction conductance by calcineurin through Cx43 phosphorylation: implications for action potential conduction
- PMID: 27757582
- PMCID: PMC5138272
- DOI: 10.1007/s00424-016-1885-7
Regulation of gap junction conductance by calcineurin through Cx43 phosphorylation: implications for action potential conduction
Abstract
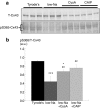
Cardiac arrhythmias are associated with raised intracellular [Ca2+] and slowed action potential conduction caused by reduced gap junction (GJ) electrical conductance (Gj). Ventricular GJs are composed of connexin proteins (Cx43), with Gj determined by Cx43 phosphorylation status. Connexin phosphorylation is an interplay between protein kinases and phosphatases but the precise pathways are unknown. We aimed to identify key Ca2+-dependent phosphorylation sites on Cx43 that regulate cardiac gap junction conductance and action potential conduction velocity. We investigated the role of the Ca2+-dependent phosphatase, calcineurin. Intracellular [Ca2+] was raised in guinea-pig myocardium by a low-Na solution or increased stimulation. Conduction velocity and Gj were measured in multicellular strips. Phosphorylation of Cx43 serine residues (S365 and S368) and of the intermediary regulator I1 at threonine35 was measured by Western blot. Measurements were made in the presence and absence of inhibitors to calcineurin, I1 or protein phosphatase-1 and phosphatase-2.Raised [Ca2+]i decreased Gj, reduced Cx43 phosphorylation at S365 and increased it at S368; these changes were reversed by calcineurin inhibitors. Cx43-S368 phosphorylation was reversed by the protein kinase C inhibitor chelerythrine. Raised [Ca2+]i also decreased I1 phosphorylation, also prevented by calcineurin inhibitors, to increase activity of the Ca2+-independent phosphatase, PPI. The PP1 inhibitor, tautomycin, prevented Cx43-365 dephosphorylation, Cx43-S368 phosphorylation and Gj reduction in raised [Ca2+]i. PP2A had no role. Conduction velocity was reduced by raised [Ca2+]i and reversed by calcineurin inhibitors. Reduced action potential conduction and Gj in raised [Ca2+] are regulated by calcineurin-dependent Cx43-S365 phosphorylation, leading to Cx43-S368 dephosphorylation. The calcineurin action is indirect, via I1 dephosphorylation and subsequent activation of PP1.
Keywords: Calcineurin; Conduction velocity; Connexin 43; Gap junction conductance.
Figures





Similar articles
-
Calcineurin-dependent regulation of gap junction conductance and connexin phosphorylation in guinea pig left atrium.Pflugers Arch. 2023 May;475(5):583-593. doi: 10.1007/s00424-023-02798-9. Epub 2023 Mar 14. Pflugers Arch. 2023. PMID: 36917272 Free PMC article.
-
Cyclic Nucleotides Differentially Regulate Cx43 Gap Junction Function in Uterine Artery Endothelial Cells From Pregnant Ewes.Hypertension. 2017 Aug;70(2):401-411. doi: 10.1161/HYPERTENSIONAHA.117.09113. Epub 2017 May 30. Hypertension. 2017. PMID: 28559397 Free PMC article.
-
Phosphorylation at Connexin43 Serine-368 Is Necessary for Myocardial Conduction During Metabolic Stress.J Cardiovasc Electrophysiol. 2016 Jan;27(1):110-9. doi: 10.1111/jce.12833. Epub 2015 Oct 13. J Cardiovasc Electrophysiol. 2016. PMID: 26459193 Free PMC article.
-
Serine-threonine protein phosphatase regulation of Cx43 dephosphorylation in arrhythmogenic disorders.Cell Signal. 2021 Oct;86:110070. doi: 10.1016/j.cellsig.2021.110070. Epub 2021 Jul 2. Cell Signal. 2021. PMID: 34217833 Free PMC article. Review.
-
[Remodeling of cardiac gap junctions and arrhythmias].Sheng Li Xue Bao. 2011 Dec 25;63(6):586-92. Sheng Li Xue Bao. 2011. PMID: 22193455 Review. Chinese.
Cited by
-
Role of Connexin 43 phosphorylation on Serine-368 by PKC in cardiac function and disease.Front Cardiovasc Med. 2023 Jan 12;9:1080131. doi: 10.3389/fcvm.2022.1080131. eCollection 2022. Front Cardiovasc Med. 2023. PMID: 36712244 Free PMC article. Review.
-
miR-133: A Suppressor of Cardiac Remodeling?Front Pharmacol. 2018 Aug 17;9:903. doi: 10.3389/fphar.2018.00903. eCollection 2018. Front Pharmacol. 2018. PMID: 30174600 Free PMC article. Review.
-
Novel approach to temozolomide resistance in malignant glioma: connexin43-directed therapeutics.Curr Opin Pharmacol. 2018 Aug;41:79-88. doi: 10.1016/j.coph.2018.05.002. Epub 2018 May 24. Curr Opin Pharmacol. 2018. PMID: 29803991 Free PMC article. Review.
-
Mitochondrial Dysfunction Increases Arrhythmic Triggers and Substrates; Potential Anti-arrhythmic Pharmacological Targets.Front Cardiovasc Med. 2021 Feb 15;8:646932. doi: 10.3389/fcvm.2021.646932. eCollection 2021. Front Cardiovasc Med. 2021. PMID: 33659284 Free PMC article. Review.
-
Enhancement of Cardiac Store Operated Calcium Entry (SOCE) within Novel Intercalated Disk Microdomains in Arrhythmic Disease.Sci Rep. 2019 Jul 15;9(1):10179. doi: 10.1038/s41598-019-46427-x. Sci Rep. 2019. PMID: 31308393 Free PMC article.
References
-
- Beardslee MA, Lerner DL, Tadros PN, Laing JG, Beyer EC, Yamada KA, Kléber AG, Schuessler RB, Saffitz JE. Dephosphorylation and intracellular redistribution of ventricular connexin43 during electrical uncoupling induced by ischemia. Circ Res. 2000;87:656–662. doi: 10.1161/01.RES.87.8.656. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

