Ciclopirox enhances pancreatic islet health by modulating the unfolded protein response in diabetes
- PMID: 27757583
- PMCID: PMC5161347
- DOI: 10.1007/s00424-016-1887-5
Ciclopirox enhances pancreatic islet health by modulating the unfolded protein response in diabetes
Abstract
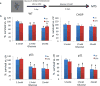
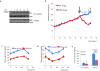
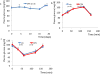
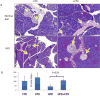
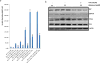
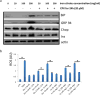
Pancreatic dysfunction during diabetes is linked to the induction of endoplasmic reticulum (ER) stress on pancreatic beta (β) cells. Our laboratory recently discovered that p21 protects from diabetes by modifying the outcome of ER stress response. In the present study, we explored the antidiabetic activity of ciclopirox (CPX), an iron chelator and recently described activator of p21 expression. The effects of CPX in beta cell survival and function were assessed in cultured islets in vitro as well as in diabetic mice in vivo. The consequences of CPX in high glucose-induced insulin release and reactive oxygen species (ROS) production were also evaluated. Islet survival assays confirmed the significance of p21 in the regulation of glucotoxicity and suggested that CPX counteracts glucotoxicity in a manner that depends on p21. In vivo, administration of CPX in wild-type (WT) diabetic mice restored glucose homeostasis. In WT-cultured islets, CPX suppressed the expression of ER stress markers BiP, GRP94, and CHOP and reduced the levels of ROS during culture at high glucose. This reduction of ER stress may be associated with the ability of CPX to inhibit insulin release. Iron citrate stimulated insulin release, which was inhibited by CPX that functions as an iron chelator. It is conceivable that inhibition of insulin production constrains ER stress in islets promoting their survival and thus protecting from diabetes in vivo. This unfolded protein response (UPR)-antagonizing activity of CPX suggests application for the management not only of diabetes but also of other conditions related to ER stress.
Keywords: Beta cells; Chaperone; Glucose; Glucotoxicity; Unfolded protein response.
Figures


References
-
- Blagosklonny MV. Are p27 and p21 cytoplasmic oncoproteins? Cell Cycle. 2002;1:391–3. - PubMed
-
- Dioufa N, Chatzistamou I, Farmaki E, Papavassiliou AG, Kiaris H. P53 Antagonizes the Unfolded Protein Response and Inhibits Ground Glass Hepatocyte Development During Endoplasmic Reticulum Stress. Experimental Biology and Medicine (Maywood, N.J) 2012;237:1173–80. - PubMed
-
- Erion KA, Ferrante T, Corkey B, Deeney J. Iron stimulates insulin secretion in clonal pancreatic β-cells and dissociated rat islets. FASEB J. 2013;27 1010.13.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

