2',3'-O-Substituted ATP derivatives as potent antagonists of purinergic P2X3 receptors and potential analgesic agents
- PMID: 27757785
- PMCID: PMC5334199
- DOI: 10.1007/s11302-016-9539-y
2',3'-O-Substituted ATP derivatives as potent antagonists of purinergic P2X3 receptors and potential analgesic agents
Abstract
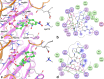
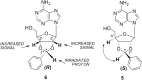
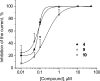
Blocking membrane currents evoked by the activation of purinergic P2X3 receptors localized on nociceptive neurons represents a promising strategy for the development of agents useful for the treatment of chronic pain conditions. Among compounds endowed with such antagonistic action, 2',3'-O-(2,4,6-trinitrophenyl)-ATP (TNP-ATP) is an ATP analogue, whose inhibitory activity on P2X receptors has been previously reported. Based on the results of molecular modelling studies performed with homology models of the P2X3 receptor, novel adenosine nucleotide analogues bearing cycloalkyl or arylalkyl substituents replacing the trinitrophenyl moiety of TNP-ATP were designed and synthesized. These new compounds were functionally evaluated on native P2X3 receptors from mouse trigeminal ganglion (TG) sensory neurons using patch clamp recordings under voltage clamp configuration. Our data show that some of these molecules are potent (nanomolar range) and reversible inhibitors of P2X3 receptors, without any apparent effect on trigeminal GABAA and 5-HT3 receptors, whose membrane currents were unaffected by the tested compounds.
Keywords: ATP derivatives; Molecular modelling; P2X3 receptor antagonists; Patch clamp; Purine derivatives; Purinergic receptors.
Conflict of interest statement
Conflict of Interest
The authors declare that they have no conflict of interest.
Figures







Similar articles
-
Evaluation of adenine as scaffold for the development of novel P2X3 receptor antagonists.Eur J Med Chem. 2013 Jul;65:41-50. doi: 10.1016/j.ejmech.2013.04.037. Epub 2013 Apr 24. Eur J Med Chem. 2013. PMID: 23688699
-
Inefficient constitutive inhibition of P2X3 receptors by brain natriuretic peptide system contributes to sensitization of trigeminal sensory neurons in a genetic mouse model of familial hemiplegic migraine.Mol Pain. 2016 May 12;12:1744806916646110. doi: 10.1177/1744806916646110. Print 2016. Mol Pain. 2016. PMID: 27175010 Free PMC article.
-
Differential expression and pharmacology of native P2X receptors in rat and primate sensory neurons.J Neurosci. 2012 Aug 22;32(34):11890-6. doi: 10.1523/JNEUROSCI.0698-12.2012. J Neurosci. 2012. PMID: 22915129 Free PMC article.
-
[P2x3-receptor desensitization as an alternative mechanism of analgesia].Fiziol Zh (1994). 2013;59(2):104-10. Fiziol Zh (1994). 2013. PMID: 23828978 Review. Ukrainian.
-
P2X3-Containing Receptors as Targets for the Treatment of Chronic Pain.Neurotherapeutics. 2020 Jul;17(3):826-838. doi: 10.1007/s13311-020-00934-2. Epub 2020 Oct 2. Neurotherapeutics. 2020. PMID: 33009633 Free PMC article. Review.
Cited by
-
P2X3 Receptor Ligands: Structural Features and Potential Therapeutic Applications.Front Pharmacol. 2021 Apr 13;12:653561. doi: 10.3389/fphar.2021.653561. eCollection 2021. Front Pharmacol. 2021. PMID: 33927627 Free PMC article. No abstract available.
-
A shared mechanism for TNP-ATP recognition by members of the P2X receptor family.Comput Struct Biotechnol J. 2023 Dec 7;23:295-308. doi: 10.1016/j.csbj.2023.12.005. eCollection 2024 Dec. Comput Struct Biotechnol J. 2023. PMID: 38173879 Free PMC article.
-
Purinergic Ligands as Potential Therapeutic Tools for the Treatment of Inflammation-Related Intestinal Diseases.Front Pharmacol. 2018 Mar 14;9:212. doi: 10.3389/fphar.2018.00212. eCollection 2018. Front Pharmacol. 2018. PMID: 29593540 Free PMC article. Review.
-
Treatment of chronic neuropathic pain: purine receptor modulation.Pain. 2020 Jul;161(7):1425-1441. doi: 10.1097/j.pain.0000000000001857. Pain. 2020. PMID: 32187120 Free PMC article.
-
Investigation on 2',3'-O-Substituted ATP Derivatives and Analogs as Novel P2X3 Receptor Antagonists.ACS Med Chem Lett. 2018 Dec 26;10(4):493-498. doi: 10.1021/acsmedchemlett.8b00524. eCollection 2019 Apr 11. ACS Med Chem Lett. 2018. PMID: 30996785 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

