Role of C16, angiopoietin-1 and regeneration gene protein 2 in attenuating inflammation in an experimental rat model of autoimmune encephalomyelitis
- PMID: 27757964
- PMCID: PMC5192791
- DOI: 10.1111/joa.12541
Role of C16, angiopoietin-1 and regeneration gene protein 2 in attenuating inflammation in an experimental rat model of autoimmune encephalomyelitis
Abstract
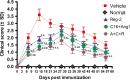
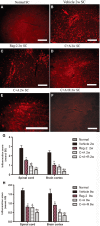
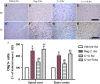
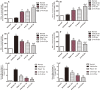
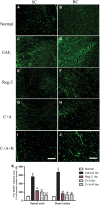
Multiple sclerosis (MS) is a chronic neurological disorder that affects the central nervous system (CNS), and results in CNS inflammation and damage to myelin. In this study, we examined the possible synergistic effects of C16, angiopoietin-1 (Ang-1) and regeneration gene protein 2 (Reg-2) in alleviating inflammation in an acute experimental autoimmune encephalomyelitis (EAE) model. We employed multiple histological, morphological and iconographic assays to examine the effect of those drugs on disease onset, clinical scores and behavioral deficits. Our results demonstrated that triple combination therapy was more efficient than the monotherapy in EAE treatment. The triple therapy significantly delayed the onset of motor symptoms, reduced disease severity, attenuated inflammatory cell infiltration and suppressed the secretion of proinflammatory cytokines. Additionally, treatment increased anti-inflammatory cytokines expression, inhibited reactive astrocytes proliferation, reduced demyelination and axonal loss, and finally reduced the neural death. Specifically, Reg-2 administration rescued oligodendrocytes and neuronal axons mainly by direct neurotrophic effects, while C16+Ang-1 (C+A) mainly improved the inflammatory milieu. In conclusion, our study suggests a possible synergistic effect through targeting a variety of pathways in relieving the clinical symptoms of inflammation in acute EAE model. Therefore, using molecules that target different molecular pathways can be beneficial for exploring novel therapeutic approaches for MS treatment.
Keywords: C16; angiopoietin-1; complementary effects; encephalomyelitis; regeneration gene protein 2.
© 2016 Anatomical Society.
Figures





Similar articles
-
Intravenous C16 and angiopoietin-1 improve the efficacy of placenta-derived mesenchymal stem cell therapy for EAE.Sci Rep. 2018 Mar 15;8(1):4649. doi: 10.1038/s41598-018-22867-9. Sci Rep. 2018. PMID: 29545630 Free PMC article.
-
Angiopoietin-1 and C16 Peptide Attenuate Vascular and Inflammatory Responses in Experimental Allergic Encephalomyelitis.CNS Neurol Disord Drug Targets. 2016;15(4):496-513. doi: 10.2174/1871527314666150821112546. CNS Neurol Disord Drug Targets. 2016. PMID: 26553158
-
C16 peptide and angiopoietin-1 alleviate the side effects of glucocorticoids in a rat multiple sclerosis model.Life Sci. 2025 Feb 15;363:123402. doi: 10.1016/j.lfs.2025.123402. Epub 2025 Jan 17. Life Sci. 2025. PMID: 39828227
-
C16 peptide shown to prevent leukocyte infiltration and alleviate detrimental inflammation in acute allergic encephalomyelitis model.Neuropharmacology. 2013 Jul;70:83-99. doi: 10.1016/j.neuropharm.2013.01.010. Epub 2013 Jan 22. Neuropharmacology. 2013. PMID: 23352465
-
Antidepressants on Multiple Sclerosis: A Review of In Vitro and In Vivo Models.Front Immunol. 2021 May 20;12:677879. doi: 10.3389/fimmu.2021.677879. eCollection 2021. Front Immunol. 2021. PMID: 34093579 Free PMC article. Review.
Cited by
-
Combined treatment with C16 peptide and angiopoietin-1 confers neuroprotection and reduces inflammation in 3-nitropropionic acid-induced dystonia mice.Aging (Albany NY). 2021 Jul 29;13(14):19048-19063. doi: 10.18632/aging.203354. Epub 2021 Jul 29. Aging (Albany NY). 2021. PMID: 34326273 Free PMC article.
-
C16 Peptide Promotes Vascular Growth and Reduces Inflammation in a Neuromyelitis Optica Model.Front Pharmacol. 2019 Dec 3;10:1373. doi: 10.3389/fphar.2019.01373. eCollection 2019. Front Pharmacol. 2019. PMID: 31849648 Free PMC article.
-
Combination Treatment of C16 Peptide and Angiopoietin-1 Alleviates Neuromyelitis Optica in an Experimental Model.Mediators Inflamm. 2018 Feb 18;2018:4187347. doi: 10.1155/2018/4187347. eCollection 2018. Mediators Inflamm. 2018. PMID: 29670463 Free PMC article.
-
Angiopoietin-1 and ανβ3 integrin peptide promote the therapeutic effects of L-serine in an amyotrophic lateral sclerosis/Parkinsonism dementia complex model.Aging (Albany NY). 2018 Nov 25;10(11):3507-3527. doi: 10.18632/aging.101661. Aging (Albany NY). 2018. PMID: 30476904 Free PMC article.
-
Adjusting vascular permeability, leukocyte infiltration, and microglial cell activation to rescue dopaminergic neurons in rodent models of Parkinson's disease.NPJ Parkinsons Dis. 2021 Oct 8;7(1):91. doi: 10.1038/s41531-021-00233-3. NPJ Parkinsons Dis. 2021. PMID: 34625569 Free PMC article.
References
-
- Albrecht PJ, Enterline JC, Cromer J, et al. (2007) CNTF‐activated astrocytes release a soluble trophic activity for oligodendrocyte progenitors. Neurochem Res 32, 263–271. - PubMed
-
- Amadio S, Pluchino S, Brini E, et al. (2006) Motor evoked potentials in a mouse model of chronic multiple sclerosis. Muscle Nerve 33, 265–273. - PubMed
-
- Baker D, Amor S (2014) Experimental autoimmune encephalomyelitis is a good model of multiple sclerosis if used wisely. Mult Scler Relat Disord 3, 555–564. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

