Recent advances in mass spectrometric analysis of glycoproteins
- PMID: 27757981
- PMCID: PMC5221507
- DOI: 10.1002/elps.201600357
Recent advances in mass spectrometric analysis of glycoproteins
Abstract
Glycosylation is one of the most common posttranslational modifications of proteins that plays essential roles in various biological processes, including protein folding, host-pathogen interaction, immune response, and inflammation and aberrant protein glycosylation is a well-known event in various disease states including cancer. As a result, it is critical to develop rapid and sensitive methods for the analysis of abnormal glycoproteins associated with diseases. Mass spectrometry (MS) in conjunction with different separation methods, such as capillary electrophoresis (CE), ion mobility (IM), and high performance liquid chromatography (HPLC) has become a popular tool for glycoprotein analysis, providing highly informative fragments for structural identification of glycoproteins. This review provides an overview of the developments and accomplishments in the field of glycomics and glycoproteomics reported between 2014 and 2016.
Keywords: Biomedical applications; Enrichment; Glycomics; Glycoproteomics; Mass spectrometry; Purification.
© 2016 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures


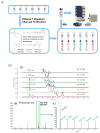

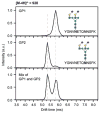
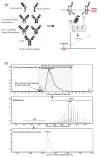
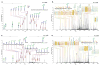
Similar articles
-
Current landscape of protein glycosylation analysis and recent progress toward a novel paradigm of glycoscience research.J Pharm Biomed Anal. 2016 Oct 25;130:273-300. doi: 10.1016/j.jpba.2016.07.015. Epub 2016 Jul 17. J Pharm Biomed Anal. 2016. PMID: 27461579 Review.
-
Quantitative glycomics strategies.Mol Cell Proteomics. 2013 Apr;12(4):874-84. doi: 10.1074/mcp.R112.026310. Epub 2013 Jan 16. Mol Cell Proteomics. 2013. PMID: 23325767 Free PMC article. Review.
-
Analysis of N- and O-linked site-specific glycosylation by ion mobility mass spectrometry: State of the art and future directions.Proteomics. 2024 Jun;24(12-13):e2300281. doi: 10.1002/pmic.202300281. Epub 2024 Jan 3. Proteomics. 2024. PMID: 38171879 Review.
-
Recent advances in mass spectrometry-based glycoproteomics.Adv Protein Chem Struct Biol. 2014;95:71-123. doi: 10.1016/B978-0-12-800453-1.00003-8. Adv Protein Chem Struct Biol. 2014. PMID: 24985770 Review.
-
Advances in LC-MS/MS-based glycoproteomics: getting closer to system-wide site-specific mapping of the N- and O-glycoproteome.Biochim Biophys Acta. 2014 Sep;1844(9):1437-52. doi: 10.1016/j.bbapap.2014.05.002. Epub 2014 May 12. Biochim Biophys Acta. 2014. PMID: 24830338 Review.
Cited by
-
Potential Plasticity of the Mannoprotein Repertoire Associated to Mycobacterium tuberculosis Virulence Unveiled by Mass Spectrometry-Based Glycoproteomics.Molecules. 2020 May 18;25(10):2348. doi: 10.3390/molecules25102348. Molecules. 2020. PMID: 32443484 Free PMC article.
-
N-Glycomics of Human Erythrocytes.Int J Mol Sci. 2021 Jul 28;22(15):8063. doi: 10.3390/ijms22158063. Int J Mol Sci. 2021. PMID: 34360826 Free PMC article.
-
MALDI Mass Spectrometry Imaging of N-Linked Glycans in Cancer Tissues.Adv Cancer Res. 2017;134:85-116. doi: 10.1016/bs.acr.2016.11.009. Epub 2016 Dec 20. Adv Cancer Res. 2017. PMID: 28110657 Free PMC article. Review.
-
Glycosylation of HDL-Associated Proteins and Its Implications in Cardiovascular Disease Diagnosis, Metabolism and Function.Front Cardiovasc Med. 2022 May 27;9:928566. doi: 10.3389/fcvm.2022.928566. eCollection 2022. Front Cardiovasc Med. 2022. PMID: 35694676 Free PMC article. Review.
-
Quantification of Protein Glycosylation Using Nanopores.Nano Lett. 2022 Jul 13;22(13):5357-5364. doi: 10.1021/acs.nanolett.2c01338. Epub 2022 Jun 29. Nano Lett. 2022. PMID: 35766994 Free PMC article.
References
-
- Mechref Y, Madera M, Novotny MV. Methods Mol Biol. 2008;424:373–96. - PubMed
-
- Kolli V, Schumacher KN, Dodds ED. Bioanalysis. 2015;7:113–31. - PubMed
-
- Leymarie N, Griffin PJ, Jonscher K, Kolarich D, Orlando R, McComb M, Zaia J, Aguilan J, Alley WR, Altmann F, Ball LE, Basumallick L, Bazemore-Walker CR, Behnken H, Blank MA, Brown KJ, Bunz SC, Cairo CW, Cipollo JF, Daneshfar R, Desaire H, Drake RR, Go EP, Goldman R, Gruber C, Halim A, Hathout Y, Hensbergen PJ, Horn DM, Hurum D, Jabs W, Larson G, Ly M, Mann BF, Marx K, Mechref Y, Meyer B, Moginger U, Neusubeta C, Nilsson J, Novotny MV, Nyalwidhe JO, Packer NH, Pompach P, Reiz B, Resemann A, Rohrer JS, Ruthenbeck A, Sanda M, Schulz JM, Schweiger-Hufnagel U, Sihlbom C, Song E, Staples GO, Suckau D, Tang H, Thaysen-Andersen M, Viner RI, An Y, Valmu L, Wada Y, Watson M, Windwarder M, Whittal R, Wuhrer M, Zhu Y, Zou C. Mol Cell Proteomics. 2013;12:2935–51. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

