Immunophenotypic analysis of erythroid dysplasia in myelodysplastic syndromes. A report from the IMDSFlow working group
- PMID: 27758818
- PMCID: PMC5286938
- DOI: 10.3324/haematol.2016.147835
Immunophenotypic analysis of erythroid dysplasia in myelodysplastic syndromes. A report from the IMDSFlow working group
Abstract
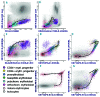
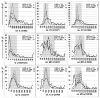
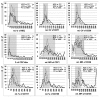
Current recommendations for diagnosing myelodysplastic syndromes endorse flow cytometry as an informative tool. Most flow cytometry protocols focus on the analysis of progenitor cells and the evaluation of the maturing myelomonocytic lineage. However, one of the most frequently observed features of myelodysplastic syndromes is anemia, which may be associated with dyserythropoiesis. Therefore, analysis of changes in flow cytometry features of nucleated erythroid cells may complement current flow cytometry tools. The multicenter study within the IMDSFlow Working Group, reported herein, focused on defining flow cytometry parameters that enable discrimination of dyserythropoiesis associated with myelodysplastic syndromes from non-clonal cytopenias. Data from a learning cohort were compared between myelodysplasia and controls, and results were validated in a separate cohort. The learning cohort comprised 245 myelodysplasia cases, 290 pathological, and 142 normal controls; the validation cohort comprised 129 myelodysplasia cases, 153 pathological, and 49 normal controls. Multivariate logistic regression analysis performed in the learning cohort revealed that analysis of expression of CD36 and CD71 (expressed as coefficient of variation), in combination with CD71 fluorescence intensity and the percentage of CD117+ erythroid progenitors provided the best discrimination between myelodysplastic syndromes and non-clonal cytopenias (specificity 90%; 95% confidence interval: 84-94%). The high specificity of this marker set was confirmed in the validation cohort (92%; 95% confidence interval: 86-97%). This erythroid flow cytometry marker combination may improve the evaluation of cytopenic cases with suspected myelodysplasia, particularly when combined with flow cytometry assessment of the myelomonocytic lineage.
Copyright© Ferrata Storti Foundation.
Figures

References
-
- Brunning R, Orazi A, Germing U, et al. Myelodysplastic syndromes/neoplasms. In: Swerdlow, et al., ed. WHO classification of Tumours and Haematopoietic and Lymphoid Tissues. Lyon: IARC; 2008
-
- Westers TM, Ireland R, Kern W, et al. Standardization of flow cytometry in myelodysplastic syndromes: a report from an international consortium and the European LeukemiaNet Working Group. Leukemia. 2012;26(7):1730–1741. - PubMed
-
- Wells DA, Benesch M, Loken MR, et al. Myeloid and monocytic dyspoiesis as determined by flow cytometric scoring in myelodysplastic syndrome correlates with the IPSS and with outcome after hematopoietic stem cell transplantation. Blood. 2003;102(1):394–403. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

