Core Binding Factor β Protects HIV, Type 1 Accessory Protein Viral Infectivity Factor from MDM2-mediated Degradation
- PMID: 27758855
- PMCID: PMC5122761
- DOI: 10.1074/jbc.M116.734673
Core Binding Factor β Protects HIV, Type 1 Accessory Protein Viral Infectivity Factor from MDM2-mediated Degradation
Abstract
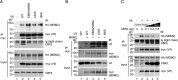
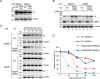
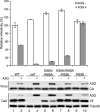
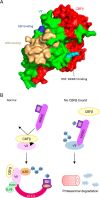
HIV, type 1 overcomes host restriction factor apolipoprotein B mRNA-editing enzyme catalytic polypeptide-like 3 (APOBEC3) proteins by organizing an E3 ubiquitin ligase complex together with viral infectivity factor (Vif) and a host transcription cofactor core binding factor β (CBFβ). CBFβ is essential for Vif to counteract APOBEC3 by enabling the recruitment of cullin 5 to the complex and increasing the steady-state level of Vif protein; however, the mechanisms by which CBFβ up-regulates Vif protein remains unclear. Because we have reported previously that mouse double minute 2 homolog (MDM2) is an E3 ligase for Vif, we hypothesized that CBFβ might protect Vif from MDM2-mediated degradation. Co-immunoprecipitation analyses showed that Vif mutants that do not bind to CBFβ preferentially interact with MDM2 and that overexpression of CBFβ disrupts the interaction between MDM2 and Vif. Knockdown of CBFβ reduced the steady-state level of Vif in MDM2-proficient cells but not in MDM2-null cells. Cycloheximide chase analyses revealed that Vif E88A/W89A, which does not interact with CBFβ, degraded faster than wild-type Vif in MDM2-proficient cells but not in MDM2-null cells, suggesting that Vif stabilization by CBFβ is mainly caused by impairing MDM2-mediated degradation. We identified Vif R93E as a Vif variant that does not bind to MDM2, and the virus with this substitution mutation was more resistant to APOBEC3G than the parental virus. Combinatory substitution of Vif residues required for CBFβ binding and MDM2 binding showed full recovery of Vif steady-state levels, supporting our hypothesis. Our data provide new insights into the mechanism of Vif augmentation by CBFβ.
Keywords: HIV; host-pathogen interaction; mouse double minute 2 homolog (MDM2); proteasome; protein-protein interaction; ubiquitin; viral protein.
© 2016 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures


References
-
- Gabuzda D. H., Li H., Lawrence K., Vasir B. S., Crawford K., and Langhoff E. (1994) Essential role of vif in establishing productive HIV-1 infection in peripheral blood T lymphocytes and monocyte/macrophages. J. Acquir. Immune Defic. Syndr. 7, 908–915 - PubMed
-
- Sheehy A. M., Gaddis N. C., Choi J. D., and Malim M. H. (2002) Isolation of a human gene that inhibits HIV-1 infection and is suppressed by the viral Vif protein. Nature 418, 646–650 - PubMed
-
- Simon J. H., Gaddis N. C., Fouchier R. A., and Malim M. H. (1998) Evidence for a newly discovered cellular anti-HIV-1 phenotype. Nat. Med. 4, 1397–1400 - PubMed
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

