Mitochondrial Activity in Human White Adipocytes Is Regulated by the Ubiquitin Carrier Protein 9/microRNA-30a Axis
- PMID: 27758866
- PMCID: PMC5114422
- DOI: 10.1074/jbc.M116.749408
Mitochondrial Activity in Human White Adipocytes Is Regulated by the Ubiquitin Carrier Protein 9/microRNA-30a Axis
Abstract
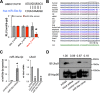
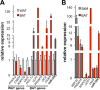
The acquisition of beige adipocyte features by white fat cells corresponds to protection against obesity-induced metabolic diseases in humans and animal models of type 2 diabetes. In adipose tissue, expression of the E2 small ubiquitin-like modifier ligase ubiquitin carrier protein 9 (Ubc9) is positively correlated with markers of insulin resistance and corresponds with impaired browning of human white adipocytes. However, the molecular regulation of Ubc9 expression in adipocytes and other cells remains unclear. In this study, we demonstrate that the mRNA and protein expression of Ubc9 are regulated by the microRNA miRNA-30a (miR-30a) in human subcutaneous adipocytes. Ubc9 and miR-30a exhibit inverse expression in adipose tissue, with miR-30a robustly elevated in brown fat. Depletion of Ubc9 by siRNA or enforced expression of a miR-30a mimic augments mitochondrial volume and respiration in human white adipocytes, reflecting features of brown fat cells. Furthermore, Ubc9 depletion induces a brown fat gene program in human subcutaneous adipocytes. Induction of the beige-selective gene program corresponds to stabilization of the PR domain-containing 16 (PRDM16) protein, an obligate transcriptional regulator of the brown/beige fat metabolic program in white adipocytes that interacts with Ubc9. Taken together, our data demonstrate a previously unappreciated molecular axis that controls browning of human white adipocytes.
Keywords: adipose tissue metabolism; bioenergetics; gene transcription; microRNA (miRNA); mitochondria; posttranscriptional regulation; respiration; tissue-specific transcription factor; transcription coregulator; transcription target gene.
© 2016 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures



Similar articles
-
miR-30a targets gene networks that promote browning of human and mouse adipocytes.Am J Physiol Endocrinol Metab. 2020 Oct 1;319(4):E667-E677. doi: 10.1152/ajpendo.00045.2020. Epub 2020 Aug 17. Am J Physiol Endocrinol Metab. 2020. PMID: 32799658 Free PMC article.
-
Ubc9 Impairs Activation of the Brown Fat Energy Metabolism Program in Human White Adipocytes.Mol Endocrinol. 2015 Sep;29(9):1320-33. doi: 10.1210/me.2015-1084. Epub 2015 Jul 20. Mol Endocrinol. 2015. PMID: 26192107 Free PMC article.
-
Reversine promotes browning of white adipocytes by suppressing miR-133a.J Cell Physiol. 2019 Apr;234(4):3800-3813. doi: 10.1002/jcp.27148. Epub 2018 Aug 21. J Cell Physiol. 2019. PMID: 30132867
-
MicroRNAs involved in the browning process of adipocytes.J Physiol Biochem. 2016 Sep;72(3):509-21. doi: 10.1007/s13105-015-0459-z. Epub 2015 Dec 22. J Physiol Biochem. 2016. PMID: 26695012 Review.
-
Role of PRDM16 in the activation of brown fat programming. Relevance to the development of obesity.Histol Histopathol. 2013 Nov;28(11):1411-25. doi: 10.14670/HH-28.1411. Epub 2013 Jun 17. Histol Histopathol. 2013. PMID: 23771475 Review.
Cited by
-
miR-30a Remodels Subcutaneous Adipose Tissue Inflammation to Improve Insulin Sensitivity in Obesity.Diabetes. 2018 Dec;67(12):2541-2553. doi: 10.2337/db17-1378. Epub 2018 Jul 12. Diabetes. 2018. PMID: 30002134 Free PMC article.
-
Physiological Approaches Targeting Cellular and Mitochondrial Pathways Underlying Adipose Organ Senescence.Int J Mol Sci. 2023 Jul 19;24(14):11676. doi: 10.3390/ijms241411676. Int J Mol Sci. 2023. PMID: 37511435 Free PMC article. Review.
-
Mitochondrial regulation and white adipose tissue homeostasis.Trends Cell Biol. 2022 Apr;32(4):351-364. doi: 10.1016/j.tcb.2021.10.008. Epub 2021 Nov 19. Trends Cell Biol. 2022. PMID: 34810062 Free PMC article. Review.
-
Transcriptional orchestration of mitochondrial homeostasis in a cellular model of PGC-1-related coactivator-dependent thyroid tumor.Oncotarget. 2018 Mar 23;9(22):15883-15894. doi: 10.18632/oncotarget.24633. eCollection 2018 Mar 23. Oncotarget. 2018. PMID: 29662614 Free PMC article.
-
Oncogene UBE2I enhances cellular invasion, migration and proliferation abilities via autophagy-related pathway resulting in poor prognosis in hepatocellular carcinoma.Am J Cancer Res. 2020 Dec 1;10(12):4178-4197. eCollection 2020. Am J Cancer Res. 2020. PMID: 33414994 Free PMC article.
References
-
- Després J. P. (2012) Body fat distribution and risk of cardiovascular disease an update. Circulation 126, 1301–1313 - PubMed
-
- Cohen P., Levy J. D., Zhang Y., Frontini A., Kolodin D. P., Svensson K. J., Lo J. C., Zeng X., Ye L., Khandekar M. J., Wu J., Gunawardana S. C., Banks A. S., Camporez J. P., Jurczak M. J., et al. (2014) Ablation of PRDM16 and beige adipose causes metabolic dysfunction and a subcutaneous to visceral fat switch. Cell 156, 304–316 - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

