Novel Molecular Interactions of Acylcarnitines and Fatty Acids with Myoglobin
- PMID: 27758871
- PMCID: PMC5122780
- DOI: 10.1074/jbc.M116.754978
Novel Molecular Interactions of Acylcarnitines and Fatty Acids with Myoglobin
Abstract
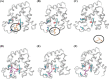
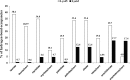
Previous research has indicated that long-chain fatty acids can bind myoglobin (Mb) in an oxygen-dependent manner. This suggests that oxy-Mb may play an important role in fuel delivery in Mb-rich muscle fibers (e.g. type I fibers and cardiomyocytes), and raises the possibility that Mb also serves as an acylcarnitine-binding protein. We report for the first time the putative interaction and affinity characteristics for different chain lengths of both fatty acids and acylcarnitines with oxy-Mb using molecular dynamic simulations and isothermal titration calorimetry experiments. We found that short- to medium-chain fatty acids or acylcarnitines (ranging from C2:0 to C10:0) fail to achieve a stable conformation with oxy-Mb. Furthermore, our results indicate that C12:0 is the minimum chain length essential for stable binding of either fatty acids or acylcarnitines with oxy-Mb. Importantly, the empirical lipid binding studies were consistent with structural modeling. These results reveal that: (i) the lipid binding affinity for oxy-Mb increases as the chain length increases (i.e. C12:0 to C18:1), (ii) the binding affinities of acylcarnitines are higher when compared with their respective fatty acid counterparts, and (iii) both fatty acids and acylcarnitines bind to oxy-Mb in 1:1 stoichiometry. Taken together, our results support a model in which oxy-Mb is a novel regulator of long-chain acylcarnitine and fatty acid pools in Mb-rich tissues. This has important implications for physiological fuel management during exercise, and relevance to pathophysiological conditions (e.g. fatty acid oxidation disorders and cardiac ischemia) where long-chain acylcarnitine accumulation is evident.
Keywords: isothermal titration calorimetry (ITC); lipid binding protein; lipotoxicity; molecular docking; molecular dynamics; myoglobin; polyunsaturated fatty acid (PUFA).
© 2016 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures




References
-
- Randle P. J. (1998) Regulatory interactions between lipids and carbohydrates: the glucose fatty acid cycle after 35 years. Diabetes Metab. Rev. 14, 263–283 - PubMed
-
- Chmurzyńska A. (2006) The multigene family of fatty acid-binding proteins (FABPs): function, structure and polymorphism. J. Appl. Genet. 47, 39–48 - PubMed
-
- Glatz J. F., and van der Vusse G. J. (1990) Nomenclature of fatty acid-binding proteins. Mol. Cell. Biochem. 98, 231–235 - PubMed
-
- Glatz J. F., and van der Vusse G. J. (1990) Cellular fatty acid-binding proteins: current concepts and future directions. Mol. Cell. Biochem. 98, 237–251 - PubMed
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

