Alveolar macrophage development in mice requires L-plastin for cellular localization in alveoli
- PMID: 27758872
- PMCID: PMC5159703
- DOI: 10.1182/blood-2016-03-705962
Alveolar macrophage development in mice requires L-plastin for cellular localization in alveoli
Abstract
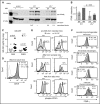
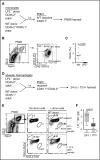
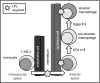
Alveolar macrophages are lung-resident sentinel cells that develop perinatally and protect against pulmonary infection. Molecular mechanisms controlling alveolar macrophage generation have not been fully defined. Here, we show that the actin-bundling protein L-plastin (LPL) is required for the perinatal development of alveolar macrophages. Mice expressing a conditional allele of LPL (CD11c.Crepos-LPLfl/fl) exhibited significant reductions in alveolar macrophages and failed to effectively clear pulmonary pneumococcal infection, showing that immunodeficiency results from reduced alveolar macrophage numbers. We next identified the phase of alveolar macrophage development requiring LPL. In mice, fetal monocytes arrive in the lungs during a late fetal stage, maturing to alveolar macrophages through a prealveolar macrophage intermediate. LPL was required for the transition from prealveolar macrophages to mature alveolar macrophages. The transition from prealveolar macrophage to alveolar macrophage requires the upregulation of the transcription factor peroxisome proliferator-activated receptor-γ (PPAR-γ), which is induced by exposure to granulocyte-macrophage colony-stimulating factor (GM-CSF). Despite abundant lung GM-CSF and intact GM-CSF receptor signaling, PPAR-γ was not sufficiently upregulated in developing alveolar macrophages in LPL-/- pups, suggesting that precursor cells were not correctly localized to the alveoli, where GM-CSF is produced. We found that LPL supports 2 actin-based processes essential for correct localization of alveolar macrophage precursors: (1) transmigration into the alveoli, and (2) engraftment in the alveoli. We thus identify a molecular pathway governing neonatal alveolar macrophage development and show that genetic disruption of alveolar macrophage development results in immunodeficiency.
© 2016 by The American Society of Hematology.
Figures





Comment in
-
Macrophage precursors PLASTed INto alveolar space.Blood. 2016 Dec 15;128(24):2750-2752. doi: 10.1182/blood-2016-10-745299. Blood. 2016. PMID: 27979865 No abstract available.
Similar articles
-
L-plastin is essential for alveolar macrophage production and control of pulmonary pneumococcal infection.Infect Immun. 2014 May;82(5):1982-93. doi: 10.1128/IAI.01199-13. Epub 2014 Mar 4. Infect Immun. 2014. PMID: 24595139 Free PMC article.
-
Inhaled GM-CSF in neonatal mice provides durable protection against bacterial pneumonia.Sci Adv. 2019 Aug 14;5(8):eaax3387. doi: 10.1126/sciadv.aax3387. eCollection 2019 Aug. Sci Adv. 2019. PMID: 31453341 Free PMC article.
-
Peroxisome proliferator-activated receptor-gamma is deficient in alveolar macrophages from patients with alveolar proteinosis.Am J Respir Cell Mol Biol. 2003 Dec;29(6):677-82. doi: 10.1165/rcmb.2003-0148OC. Epub 2003 Jun 12. Am J Respir Cell Mol Biol. 2003. PMID: 12805087
-
Gm-CSF regulates pulmonary surfactant homeostasis and alveolar macrophage-mediated innate host defense.Annu Rev Physiol. 2002;64:775-802. doi: 10.1146/annurev.physiol.64.090601.113847. Annu Rev Physiol. 2002. PMID: 11826288 Review.
-
The actin-bundling protein L-plastin supports T-cell motility and activation.Immunol Rev. 2013 Nov;256(1):48-62. doi: 10.1111/imr.12102. Immunol Rev. 2013. PMID: 24117812 Free PMC article. Review.
Cited by
-
Macrophages in health and disease.Cell. 2022 Nov 10;185(23):4259-4279. doi: 10.1016/j.cell.2022.10.007. Cell. 2022. PMID: 36368305 Free PMC article. Review.
-
PPAR-γ in Macrophages Limits Pulmonary Inflammation and Promotes Host Recovery following Respiratory Viral Infection.J Virol. 2019 Apr 17;93(9):e00030-19. doi: 10.1128/JVI.00030-19. Print 2019 May 1. J Virol. 2019. PMID: 30787149 Free PMC article.
-
Macrophage TLR4 and PAR2 Signaling: Role in Regulating Vascular Inflammatory Injury and Repair.Front Immunol. 2020 Sep 18;11:2091. doi: 10.3389/fimmu.2020.02091. eCollection 2020. Front Immunol. 2020. PMID: 33072072 Free PMC article. Review.
-
L-plastin enhances NLRP3 inflammasome assembly and bleomycin-induced lung fibrosis.Cell Rep. 2022 Mar 15;38(11):110507. doi: 10.1016/j.celrep.2022.110507. Cell Rep. 2022. PMID: 35294888 Free PMC article.
-
Lung Immune Cell Niches and the Discovery of New Cell Subtypes.Adv Sci (Weinh). 2024 Dec;11(45):e2405490. doi: 10.1002/advs.202405490. Epub 2024 Oct 14. Adv Sci (Weinh). 2024. PMID: 39401416 Free PMC article. Review.
References
-
- Kopf M, Schneider C, Nobs SP. The development and function of lung-resident macrophages and dendritic cells. Nat Immunol. 2015;16(1):36-44. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

