Systems analysis uncovers inflammatory Th/Tc17-driven modules during acute GVHD in monkey and human T cells
- PMID: 27758873
- PMCID: PMC5123196
- DOI: 10.1182/blood-2016-07-726547
Systems analysis uncovers inflammatory Th/Tc17-driven modules during acute GVHD in monkey and human T cells
Abstract
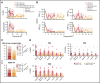
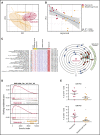
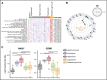
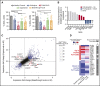
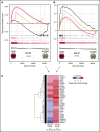
One of the central challenges of transplantation is the development of alloreactivity despite the use of multiagent immunoprophylaxis. Effective control of this immune suppression-resistant T-cell activation represents one of the key unmet needs in the fields of both solid-organ and hematopoietic stem cell transplant (HCT). To address this unmet need, we have used a highly translational nonhuman primate (NHP) model to interrogate the transcriptional signature of T cells during breakthrough acute graft-versus-host disease (GVHD) that occurs in the setting of clinically relevant immune suppression and compared this to the hyperacute GVHD, which develops in unprophylaxed or suboptimally prophylaxed transplant recipients. Our results demonstrate the complex character of the alloreactivity that develops during ongoing immunoprophylaxis and identify 3 key transcriptional hallmarks of breakthrough acute GVHD that are not observed in hyperacute GVHD: (1) T-cell persistence rather than proliferation, (2) evidence for highly inflammatory transcriptional programming, and (3) skewing toward a T helper (Th)/T cytotoxic (Tc)17 transcriptional program. Importantly, the gene coexpression profiles from human HCT recipients who developed GVHD while on immunosuppressive prophylactic agents recapitulated the patterns observed in NHP, and demonstrated an evolution toward a more inflammatory signature as time posttransplant progressed. These results strongly implicate the evolution of both inflammatory and interleukin 17-based immune pathogenesis in GVHD, and provide the first map of this evolving process in primates in the setting of clinically relevant immunomodulation. This map represents a novel transcriptomic resource for further systems-based efforts to study the breakthrough alloresponse that occurs posttransplant despite immunoprophylaxis and to develop evidence-based strategies for effective treatment of this disease.
© 2016 by The American Society of Hematology.
Figures


References
-
- Organ Procurement and Transplantation Network. Available at: http://optn.transplant.hrsa.gov/.
-
- Miller WP, Srinivasan S, Panoskaltsis-Mortari A, et al. . GVHD after haploidentical transplantation: a novel, MHC-defined rhesus macaque model identifies CD28- CD8+ T cells as a reservoir of breakthrough T-cell proliferation during costimulation blockade and sirolimus-based immunosuppression. Blood. 2010;116(24):5403-5418. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

