Destabilization of Fatty Acid Synthase by Acetylation Inhibits De Novo Lipogenesis and Tumor Cell Growth
- PMID: 27758890
- PMCID: PMC5135623
- DOI: 10.1158/0008-5472.CAN-16-1597
Destabilization of Fatty Acid Synthase by Acetylation Inhibits De Novo Lipogenesis and Tumor Cell Growth
Abstract
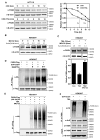
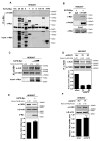
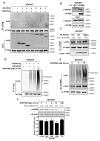
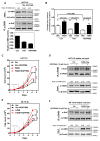
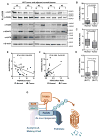
Fatty acid synthase (FASN) is the terminal enzyme in de novo lipogenesis and plays a key role in cell proliferation. Pharmacologic inhibitors of FASN are being evaluated in clinical trials for treatment of cancer, obesity, and other diseases. Here, we report a previously unknown mechanism of FASN regulation involving its acetylation by KAT8 and its deacetylation by HDAC3. FASN acetylation promoted its degradation via the ubiquitin-proteasome pathway. FASN acetylation enhanced its association with the E3 ubiquitin ligase TRIM21. Acetylation destabilized FASN and resulted in decreased de novo lipogenesis and tumor cell growth. FASN acetylation was frequently reduced in human hepatocellular carcinoma samples, which correlated with increased HDAC3 expression and FASN protein levels. Our results suggest opportunities to target FASN acetylation as an anticancer strategy. Cancer Res; 76(23); 6924-36. ©2016 AACR.
©2016 American Association for Cancer Research.
Conflict of interest statement
No potential conflict of interest was disclosed.
Figures


Similar articles
-
Acetyl-CoA-mediated autoacetylation of fatty acid synthase as a metabolic switch of de novo lipogenesis in Drosophila.Proc Natl Acad Sci U S A. 2022 Dec 6;119(49):e2212220119. doi: 10.1073/pnas.2212220119. Epub 2022 Dec 2. Proc Natl Acad Sci U S A. 2022. PMID: 36459649 Free PMC article.
-
Targeting fatty acid synthase in breast and endometrial cancer: An alternative to selective estrogen receptor modulators?Endocrinology. 2006 Sep;147(9):4056-66. doi: 10.1210/en.2006-0486. Epub 2006 Jun 29. Endocrinology. 2006. PMID: 16809439 Review.
-
Blockade of fatty acid synthase induces ubiquitination and degradation of phosphoinositide-3-kinase signaling proteins in ovarian cancer.Mol Cancer Res. 2011 Dec;9(12):1767-79. doi: 10.1158/1541-7786.MCR-10-0467. Epub 2011 Oct 4. Mol Cancer Res. 2011. PMID: 21970855
-
Differential requirement for de novo lipogenesis in cholangiocarcinoma and hepatocellular carcinoma of mice and humans.Hepatology. 2016 Jun;63(6):1900-13. doi: 10.1002/hep.28508. Epub 2016 Mar 25. Hepatology. 2016. PMID: 26910791 Free PMC article.
-
Recent advances in targeting the fatty acid biosynthetic pathway using fatty acid synthase inhibitors.Expert Opin Drug Discov. 2016 Dec;11(12):1187-1199. doi: 10.1080/17460441.2016.1245286. Epub 2016 Oct 18. Expert Opin Drug Discov. 2016. PMID: 27701891 Review.
Cited by
-
Human milk-derived extracellular vesicles alleviate high fat diet-induced non-alcoholic fatty liver disease in mice.Mol Biol Rep. 2023 Mar;50(3):2257-2268. doi: 10.1007/s11033-022-08206-2. Epub 2022 Dec 27. Mol Biol Rep. 2023. PMID: 36575319
-
Fenofibrate induces human hepatoma Hep3B cells apoptosis and necroptosis through inhibition of thioesterase domain of fatty acid synthase.Sci Rep. 2019 Mar 1;9(1):3306. doi: 10.1038/s41598-019-39778-y. Sci Rep. 2019. PMID: 30824767 Free PMC article.
-
Advanced Maternal Age-associated SIRT1 Deficiency Compromises Trophoblast Epithelial-Mesenchymal Transition through an Increase in Vimentin Acetylation.Aging Cell. 2021 Oct;20(10):e13491. doi: 10.1111/acel.13491. Epub 2021 Oct 3. Aging Cell. 2021. PMID: 34605151 Free PMC article.
-
ACLY ubiquitination by CUL3-KLHL25 induces the reprogramming of fatty acid metabolism to facilitate iTreg differentiation.Elife. 2021 Sep 7;10:e62394. doi: 10.7554/eLife.62394. Elife. 2021. PMID: 34491895 Free PMC article.
-
Exploiting E3 ubiquitin ligases to reeducate the tumor microenvironment for cancer therapy.Exp Hematol Oncol. 2023 Mar 30;12(1):34. doi: 10.1186/s40164-023-00394-2. Exp Hematol Oncol. 2023. PMID: 36998063 Free PMC article. Review.
References
-
- Gansler TS, Hardman W, 3rd, Hunt DA, Schaffel S, Hennigar RA. Increased expression of fatty acid synthase (OA-519) in ovarian neoplasms predicts shorter survival. Human pathology. 1997;28:686–92. - PubMed
-
- Hao Q, Li T, Zhang X, Gao P, Qiao P, Li S, et al. Expression and roles of fatty acid synthase in hepatocellular carcinoma. Oncology reports. 2014;32:2471–6. - PubMed
-
- Menendez JA, Mehmi I, Atlas E, Colomer R, Lupu R. Novel signaling molecules implicated in tumor-associated fatty acid synthase-dependent breast cancer cell proliferation and survival: Role of exogenous dietary fatty acids, p53-p21WAF1/CIP1, ERK1/2 MAPK, p27KIP1, BRCA1, and NF-kappaB. Int J Oncol. 2004;24:591–608. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

