SIKs control osteocyte responses to parathyroid hormone
- PMID: 27759007
- PMCID: PMC5075806
- DOI: 10.1038/ncomms13176
SIKs control osteocyte responses to parathyroid hormone
Erratum in
-
Corrigendum: SIKs control osteocyte responses to parathyroid hormone.Nat Commun. 2017 Feb 22;8:14745. doi: 10.1038/ncomms14745. Nat Commun. 2017. PMID: 28224982 Free PMC article. No abstract available.
Abstract
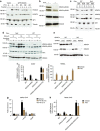
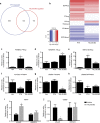
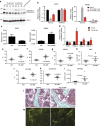
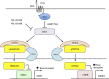
Parathyroid hormone (PTH) activates receptors on osteocytes to orchestrate bone formation and resorption. Here we show that PTH inhibition of SOST (sclerostin), a WNT antagonist, requires HDAC4 and HDAC5, whereas PTH stimulation of RANKL, a stimulator of bone resorption, requires CRTC2. Salt inducible kinases (SIKs) control subcellular localization of HDAC4/5 and CRTC2. PTH regulates both HDAC4/5 and CRTC2 localization via phosphorylation and inhibition of SIK2. Like PTH, new small molecule SIK inhibitors cause decreased phosphorylation and increased nuclear translocation of HDAC4/5 and CRTC2. SIK inhibition mimics many of the effects of PTH in osteocytes as assessed by RNA-seq in cultured osteocytes and following in vivo administration. Once daily treatment with the small molecule SIK inhibitor YKL-05-099 increases bone formation and bone mass. Therefore, a major arm of PTH signalling in osteocytes involves SIK inhibition, and small molecule SIK inhibitors may be applied therapeutically to mimic skeletal effects of PTH.
Conflict of interest statement
Y.L. and N.G. are co-inventors of YKL-04-114, YKL-05-093 and YKL-05-099. H.M.K. receives grant support from Amgen, and has received consulting honorariums from Amgen and Novartis. M.B. receives grant support from Amgen and Merck, and serves on scientific advisory boards for Merck and Eli Lilly. T.S., R.X., H.M.K., and M.N.W. declare that patents have been filed for the therapeutic application of SIK inhibitors. The remaining authors declare no competing financial interests.
Figures





Comment in
-
Parathyroid function: Action of parathyroid hormone in osteocytes.Nat Rev Endocrinol. 2017 Jan;13(1):4. doi: 10.1038/nrendo.2016.184. Epub 2016 Nov 4. Nat Rev Endocrinol. 2017. PMID: 27811941 No abstract available.
Similar articles
-
Effects of histone deacetylase inhibitor Scriptaid and parathyroid hormone on osteocyte functions and metabolism.J Biol Chem. 2019 Jun 21;294(25):9722-9733. doi: 10.1074/jbc.RA118.007312. Epub 2019 May 8. J Biol Chem. 2019. PMID: 31068415 Free PMC article.
-
Lipoprotein receptor-related protein 6 is required for parathyroid hormone-induced Sost suppression.Ann N Y Acad Sci. 2016 Jan;1364(1):62-73. doi: 10.1111/nyas.12750. Epub 2015 Apr 2. Ann N Y Acad Sci. 2016. PMID: 25847683 Free PMC article.
-
Resorption controls bone anabolism driven by parathyroid hormone (PTH) receptor signaling in osteocytes.J Biol Chem. 2013 Oct 11;288(41):29809-20. doi: 10.1074/jbc.M113.485938. Epub 2013 Aug 20. J Biol Chem. 2013. PMID: 23963454 Free PMC article.
-
Does osteocytic SOST suppression mediate PTH bone anabolism?Trends Endocrinol Metab. 2010 Apr;21(4):237-44. doi: 10.1016/j.tem.2009.12.002. Epub 2010 Jan 13. Trends Endocrinol Metab. 2010. PMID: 20074973 Review.
-
Effects of PTH on osteocyte function.Bone. 2013 Jun;54(2):250-7. doi: 10.1016/j.bone.2012.09.016. Epub 2012 Sep 24. Bone. 2013. PMID: 23017659 Free PMC article. Review.
Cited by
-
PTH, FGF-23, Klotho and Vitamin D as regulators of calcium and phosphorus: Genetics, epigenetics and beyond.Front Endocrinol (Lausanne). 2022 Sep 29;13:992666. doi: 10.3389/fendo.2022.992666. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 36246903 Free PMC article. Review.
-
Aberrant Bone Regulation in Albright Hereditary Osteodystrophy dueto Gnas Inactivation: Mechanisms and Translational Implications.Curr Osteoporos Rep. 2022 Feb;20(1):78-89. doi: 10.1007/s11914-022-00719-w. Epub 2022 Feb 28. Curr Osteoporos Rep. 2022. PMID: 35226254 Review.
-
TG-interacting factor 1 (Tgif1)-deficiency attenuates bone remodeling and blunts the anabolic response to parathyroid hormone.Nat Commun. 2019 Mar 22;10(1):1354. doi: 10.1038/s41467-019-08778-x. Nat Commun. 2019. PMID: 30902975 Free PMC article.
-
Wnt/β-catenin signaling components and mechanisms in bone formation, homeostasis, and disease.Bone Res. 2024 Jul 10;12(1):39. doi: 10.1038/s41413-024-00342-8. Bone Res. 2024. PMID: 38987555 Free PMC article. Review.
-
Regulatory mechanisms of sclerostin expression during bone remodeling.J Bone Miner Metab. 2019 Jan;37(1):9-17. doi: 10.1007/s00774-018-0971-7. Epub 2018 Oct 24. J Bone Miner Metab. 2019. PMID: 30357564 Review.
References
-
- Harvey N., Dennison E. & Cooper C. Osteoporosis: impact on health and economics. Nat. Rev. Rheumatol. 6, 99–105 (2010). - PubMed
-
- Nakashima T. et al.. Evidence for osteocyte regulation of bone homeostasis through RANKL expression. Nat. Med. 17, 1231–1234 (2011). - PubMed
-
- Baron R. & Kneissel M. WNT signaling in bone homeostasis and disease: from human mutations to treatments. Nat. Med. 19, 179–192 (2013). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

