Plasmodium vivax GPI-anchored micronemal antigen (PvGAMA) binds human erythrocytes independent of Duffy antigen status
- PMID: 27759110
- PMCID: PMC5069673
- DOI: 10.1038/srep35581
Plasmodium vivax GPI-anchored micronemal antigen (PvGAMA) binds human erythrocytes independent of Duffy antigen status
Abstract
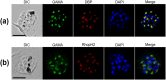
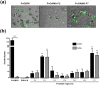
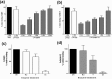
Plasmodium vivax, a major agent of malaria in both temperate and tropical climates, has been thought to be unable to infect humans lacking the Duffy (Fy) blood group antigen because this receptor is critical for erythrocyte invasion. Recent surveys in various endemic regions, however, have reported P. vivax infections in Duffy-negative individuals, suggesting that the parasite may utilize alternative receptor-ligand pairs to complete the erythrocyte invasion. Here, we identified and characterized a novel parasite ligand, Plasmodium vivax GPI-anchored micronemal antigen (PvGAMA), that bound human erythrocytes regardless of Duffy antigen status. PvGAMA was localized at the microneme in the mature schizont-stage parasites. The antibodies against PvGAMA fragments inhibited PvGAMA binding to erythrocytes in a dose-dependent manner. The erythrocyte-specific binding activities of PvGAMA were significantly reduced by chymotrypsin treatment. Thus, PvGAMA may be an adhesion molecule for the invasion of Duffy-positive and -negative human erythrocytes.
Figures



References
-
- Miller L. H., Mason S. J., Dvorak J. A., McGinniss M. H. & Rothman I. K. Erythrocyte receptors for (Plasmodium knowlesi) malaria: Duffy blood group determinants. Science 189, 561–563 (1975). - PubMed
-
- Adams J. H. et al.. The Duffy receptor family of Plasmodium knowlesi is located within the micronemes of invasive malaria merozoites. Cell 63, 141–153 (1990). - PubMed
-
- Horuk R. et al.. A receptor for the malarial parasite Plasmodium vivax: the erythrocyte chemokine receptor. Science 261, 1182–1184 (1993). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

