Thiazolidinediones for nonalcoholic steatohepatitis: A meta-analysis of randomized clinical trials
- PMID: 27759627
- PMCID: PMC5079311
- DOI: 10.1097/MD.0000000000004947
Thiazolidinediones for nonalcoholic steatohepatitis: A meta-analysis of randomized clinical trials
Abstract
The findings regarding the effects of thiazolidinediones (TZDs) in nonalcoholic steatohepatitis (NASH) patients have been inconsistent, and the assessment of different clinical variables for evaluating the effects of TZDs confound a direct comparison of the results of different randomized clinical trials (RCTs), especially with regard to lifestyle changes. In this paper, we performed a meta-analysis of randomized controlled trials to clarify the effects of TZD treatment with and without lifestyle changes on histological markers of NASH and clinical variables related to insulin resistance (IR), hyperlipidemia, and obesity. We searched the literature using the following MeSH terms: "nonalcoholic steatohepatitis," "non-alcoholic steatohepatitis," "thiazolidinedione," "pioglitazone," "rosiglitazone," "randomized," and "clinical trial." Five eligible RCTs were selected, in which patients were treated with either pioglitazone or rosiglitazone, with or without lifestyle changes. We compared the effects of TZD treatment on hepatic fibrosis, lobular inflammation, IR improvement, fasting serum insulin, adiposity, and dyslipidemia between the various studies using fixed and random effects models, and heterogeneity in clinical outcomes was assessed. Significant improvement in hepatic fibrosis did not occur among the patients treated with TZDs alone or in those who underwent both lifestyle changes and TZD therapy. Lobular inflammation decreased in NASH patients who received TZD treatment and in those who underwent both TZD therapy and lifestyle changes. Although TZD treatment resulted in no significant improvement in IR, NASH patients who underwent both lifestyle changes and TZD therapy experienced a significantly greater reduction in their fasting insulin level than that observed in the control patients, whereas patients treated with TZDs alone did not. Although TZD-treated patients experienced significantly greater weight gain than the control patients, TZD treatment had no significant impact on body-mass index, percentage of body fat, or serum levels of cholesterol and triglyceride. Our findings indicate that additional variables should be assessed to obtain a more comprehensive evaluation of the effects of TZD treatment on IR and comorbidity risk factors in NASH patients, and suggest that including lifestyle changes and additional insulin-sensitizing agents in TZD regimens might improve the benefits of TZD therapy for NASH.
Conflict of interest statement
All listed authors declare that there are no conflicts of interest.
Figures








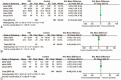
References
-
- Ekstedt M, Franzen LE, Mathiesen UL, et al. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology 2006; 44:865–873. - PubMed
-
- Paredes AH, Torres DM, Harrison SA. Nonalcoholic fatty liver disease. Clin Liver Dis 2012; 16:397–419. - PubMed
-
- Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of non-alcoholic fatty liver disease: practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology 2012; 55:2005–2023. - PubMed
-
- Karlas T, Wiegand J, Berg T. Gastrointestinal complications of obesity: non-alcoholic fatty liver disease (NAFLD) and its sequelae. Best Pract Res Clin Endocrinol Metab 2013; 27:195–208. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

