The peptide Phα1β, from spider venom, acts as a TRPA1 channel antagonist with antinociceptive effects in mice
- PMID: 27759880
- PMCID: PMC5341489
- DOI: 10.1111/bph.13652
The peptide Phα1β, from spider venom, acts as a TRPA1 channel antagonist with antinociceptive effects in mice
Abstract
Background and purpose: Peptides from venomous animals have long been important for understanding pain mechanisms and for the discovery of pain treatments. Here, we hypothesized that Phα1β, a peptide from the venom of the armed spider Phoneutria nigriventer, produces analgesia by blocking the TRPA1 channel.
Experimental approach: Cultured rat dorsal root ganglion (DRG) neurons, human fetal lung fibroblasts (IMR90) or HEK293 cells expressing the human TRPA1 (hTRPA1-HEK293), human TRPV1 (hTRPV1-HEK293) or human TRPV4 channels (hTRPV4-HEK293), were used for calcium imaging and electrophysiology. Nociceptive responses induced by TRPA1, TRPV1 or TRPV4 agonists or by bortezomib were investigated in mice.
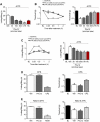
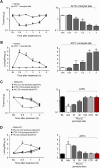
Key results: Phα1β selectively inhibited calcium responses and currents evoked by the TRPA1 agonist, allyl isothiocyanate (AITC), on hTRPA1-HEK293, IMR90 fibroblasts and DRG neurons. Phα1β did not affect calcium responses evoked by selective TRPV1 (capsaicin) or TRPV4 (GSK 1016790A) agonists on the various cell types. Intrathecal (i.t.) and intraplantar (i.pl.) administration of low doses of Phα1β (up to 300 pmol per paw) attenuated acute nociception and mechanical and cold hyperalgesia evoked by AITC (i.t. or i.pl.), without affecting responses produced by capsaicin or hypotonic solution. Notably, Phα1β abated the TRPA1-dependent neuropathic pain-like responses induced by bortezomib. In vitro and in vivo inhibition of TRPA1 by Phα1β was reproduced by a recombinant form of the peptide, CTK 01512-2.
Conclusions and implications: Phα1β and CTK 01512-2 selectively target TRPA1, but not other TRP channels. This specific action underlines the potential of Phα1β and CTK 01512-2 for pain treatment.
© 2016 The British Pharmacological Society.
Figures



References
-
- Andrade EL, Meotti FC, Calixto JB (2012). TRPA1 antagonists as potential analgesic drugs. Pharmacol Ther 133: 189–204. - PubMed
-
- Andreev YA, Kozlov SA, Koshelev SG, Ivanova EA, Monastyrnaya MM, Kozlovskaya EP et al. (2008). Analgesic compound from sea anemone <styled-content style="fixed-case"><styled-content style="italic-in-any-context">Heteractis crispa</styled-content></styled-content> is the first polypeptide inhibitor of vanilloid receptor 1 (TRPV1). J Biol Chem 283: 23914–23921. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

