Deficiency of base excision repair enzyme NEIL3 drives increased predisposition to autoimmunity
- PMID: 27760045
- PMCID: PMC5096910
- DOI: 10.1172/JCI85647
Deficiency of base excision repair enzyme NEIL3 drives increased predisposition to autoimmunity
Abstract
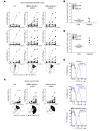
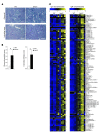
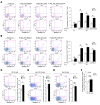
Alterations in the apoptosis of immune cells have been associated with autoimmunity. Here, we have identified a homozygous missense mutation in the gene encoding the base excision repair enzyme Nei endonuclease VIII-like 3 (NEIL3) that abolished enzymatic activity in 3 siblings from a consanguineous family. The NEIL3 mutation was associated with fatal recurrent infections, severe autoimmunity, hypogammaglobulinemia, and impaired B cell function in these individuals. The same homozygous NEIL3 mutation was also identified in an asymptomatic individual who exhibited elevated levels of serum autoantibodies and defective peripheral B cell tolerance, but normal B cell function. Further analysis of the patients revealed an absence of LPS-responsive beige-like anchor (LRBA) protein expression, a known cause of immunodeficiency. We next examined the contribution of NEIL3 to the maintenance of self-tolerance in Neil3-/- mice. Although Neil3-/- mice displayed normal B cell function, they exhibited elevated serum levels of autoantibodies and developed nephritis following treatment with poly(I:C) to mimic microbial stimulation. In Neil3-/- mice, splenic T and B cells as well as germinal center B cells from Peyer's patches showed marked increases in apoptosis and cell death, indicating the potential release of self-antigens that favor autoimmunity. These findings demonstrate that deficiency in NEIL3 is associated with increased lymphocyte apoptosis, autoantibodies, and predisposition to autoimmunity.
Figures




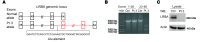

Comment in
-
Genetic cause of immune dysregulation - one gene or two?J Clin Invest. 2016 Nov 1;126(11):4065-4067. doi: 10.1172/JCI90831. Epub 2016 Oct 17. J Clin Invest. 2016. PMID: 27760052 Free PMC article.
References
-
- Emlen W, Niebur J, Kadera R. Accelerated in vitro apoptosis of lymphocytes from patients with systemic lupus erythematosus. J Immunol. 1994;152(7):3685–3692. - PubMed
MeSH terms
Substances
Grants and funding
- RC2 HL102923/HL/NHLBI NIH HHS/United States
- UC2 HL102926/HL/NHLBI NIH HHS/United States
- UC2 HL103010/HL/NHLBI NIH HHS/United States
- K08 AI114968/AI/NIAID NIH HHS/United States
- RC2 HL102926/HL/NHLBI NIH HHS/United States
- UL1 TR001863/TR/NCATS NIH HHS/United States
- RC2 HL102924/HL/NHLBI NIH HHS/United States
- P01 AI061093/AI/NIAID NIH HHS/United States
- R01 AI042269/AI/NIAID NIH HHS/United States
- UC2 HL102923/HL/NHLBI NIH HHS/United States
- UC2 HL102924/HL/NHLBI NIH HHS/United States
- RC2 HL103010/HL/NHLBI NIH HHS/United States
- RC2 HL102925/HL/NHLBI NIH HHS/United States
- UC2 HL102925/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

