Protective Efficacy of Plasmodium vivax Radiation-Attenuated Sporozoites in Colombian Volunteers: A Randomized Controlled Trial
- PMID: 27760143
- PMCID: PMC5070852
- DOI: 10.1371/journal.pntd.0005070
Protective Efficacy of Plasmodium vivax Radiation-Attenuated Sporozoites in Colombian Volunteers: A Randomized Controlled Trial
Abstract
Background: Immunizing human volunteers by mosquito bite with radiation-attenuated Plasmodium falciparum sporozoites (RAS) results in high-level protection against infection. Only two volunteers have been similarly immunized with P. vivax (Pv) RAS, and both were protected. A phase 2 controlled clinical trial was conducted to assess the safety and protective efficacy of PvRAS immunization.
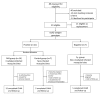
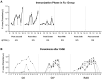
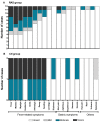
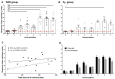
Methodology/principal findings: A randomized, single-blinded trial was conducted. Duffy positive (Fy+; Pv susceptible) individuals were enrolled: 14 received bites from irradiated (150 ± 10 cGy) Pv-infected Anopheles mosquitoes (RAS) and 7 from non-irradiated non-infected mosquitoes (Ctl). An additional group of seven Fy- (Pv refractory) volunteers was immunized with bites from non-irradiated Pv-infected mosquitoes. A total of seven immunizations were carried out at mean intervals of nine weeks. Eight weeks after last immunization, a controlled human malaria infection (CHMI) with non-irradiated Pv-infected mosquitoes was performed. Nineteen volunteers completed seven immunizations (12 RAS, 2 Ctl, and 5 Fy-) and received a CHMI. Five of 12 (42%) RAS volunteers were protected (receiving a median of 434 infective bites) compared with 0/2 Ctl. None of the Fy- volunteers developed infection by the seventh immunization or after CHMI. All non-protected volunteers developed symptoms 8-13 days after CHMI with a mean pre-patent period of 12.8 days. No serious adverse events related to the immunizations were observed. Specific IgG1 anti-PvCS response was associated with protection.
Conclusion: Immunization with PvRAS was safe, immunogenic, and induced sterile immunity in 42% of the Fy+ volunteers. Moreover, Fy- volunteers were refractory to Pv malaria.
Trial registration: Identifier: NCT01082341.
Conflict of interest statement
I have read the journal's policy and the authors of this manuscript have the following competing interests: TLR is a salaried, full time employee of Sanaria Inc., the developer and sponsor of Sanaria PfSPZ vaccine. JT and JO are full time employee of Asoclinic Inmunología LTDA and Centro Médico Imbanaco, respectively. The other authors have declared that no competing interests exist.
Figures


References
-
- World Health Organization. World Malaria Report 2015. Geneva, Switzerland: World Health Organization, 2015.
-
- World Health Organization. Immunization, vaccines and biologicals SAGE Meeting of October 2015: World Healt Organization; 2015 [October 26, 2015]. Available from: http://www.who.int/immunization/sage/meetings/2015/october/en/.
-
- Clyde DF, Most H, McCarthy VC, Vanderberg JP. Immunization of man against sporozite-induced falciparum malaria. Am J Med Sci. 1973;266(3):169–77. - PubMed
-
- Rieckmann KH, Carson PE, Beaudoin RL, Cassells JS, Sell KW. Letter: Sporozoite induced immunity in man against an Ethiopian strain of Plasmodium falciparum. Trans R Soc Trop Med Hyg. 1974;68(3):258–9. - PubMed
-
- Clyde DF. Immunization of man against falciparum and vivax malaria by use of attenuated sporozoites. Am J Trop Med Hyg. 1975;24(3):397–401. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

