Role of PXR in Hepatic Cancer: Its Influences on Liver Detoxification Capacity and Cancer Progression
- PMID: 27760163
- PMCID: PMC5070842
- DOI: 10.1371/journal.pone.0164087
Role of PXR in Hepatic Cancer: Its Influences on Liver Detoxification Capacity and Cancer Progression
Abstract
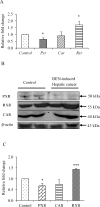
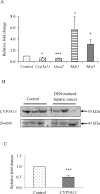
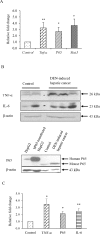
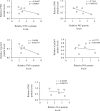
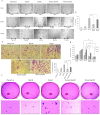
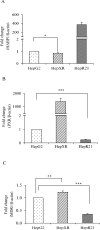
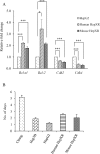
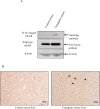
The role of nuclear receptor PXR in detoxification and clearance of xenobiotics and endobiotics is well-established. However, its projected role in hepatic cancer is rather illusive where its expression is reported altered in different cancers depending on the tissue-type and microenvironment. The expression of PXR, its target genes and their biological or clinical significance have not been examined in hepatic cancer. In the present study, by generating DEN-induced hepatic cancer in mice, we report that the expression of PXR and its target genes CYP3A11 and GSTa2 are down-regulated implying impairment of hepatic detoxification capacity. A higher state of inflammation was observed in liver cancer tissues as evident from upregulation of inflammatory cytokines IL-6 and TNF-α along with NF-κB and STAT3. Our data in mouse model suggested a negative correlation between down-regulation of PXR and its target genes with that of higher expression of inflammatory proteins (like IL-6, TNF-α, NF-κB). In conjunction, our findings with relevant cell culture based assays showed that higher expression of PXR is involved in reduction of tumorigenic potential in hepatic cancer. Overall, the findings suggest that inflammation influences the expression of hepatic proteins important in drug metabolism while higher PXR level reduces tumorigenic potential in hepatic cancer.
Conflict of interest statement
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Figures
References
-
- Heng Jiang KC, He Jianming, Pan Feng, Li Jianjun, Chen Jianfang,Chen Wensheng, Liang Houjie. Association of Pregnane X Receptor with multidrug resistance-related protein 3 and its role in human colon cancer chemoresistance. J Gastrointest Surg. 2009. 13:1831–8. 10.1007/s11605-009-0964-x - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

