Prolyl Oligopeptidase Inhibition Attenuates Steatosis in the L02 Human Liver Cell Line
- PMID: 27760195
- PMCID: PMC5070736
- DOI: 10.1371/journal.pone.0165224
Prolyl Oligopeptidase Inhibition Attenuates Steatosis in the L02 Human Liver Cell Line
Abstract
Background: Prolyl oligopeptidase (POP) is a serine endopeptidase that is widely distributed in vivo, particularly in the liver. Significant changes in functional mitochondrial proteins involved with mitochondrial oxidoreductases/transporters and nucleic acid binding proteins were observed after POP inhibition in the liver, which suggested a role of POP in regulating liver energy metabolism. Steatosis in nonalcoholic fatty liver disease (NAFLD) is associated with disturbances in lipid and energy metabolism in hepatocytes. Here, we aimed to study the effect of POP on hepatocyte steatosis.
Methods: The human liver cell line L02 was used to investigate the biological effects of POP. An in vitro cell model of steatosis was successfully induced with oleic acid and palmitic acid. L02 cells were also subjected to S17092 (a POP inhibitor) at different concentrations for 24 or 48 h. Ac-SDKP levels and POP activity were measured to assess the rate of inhibition of POP by S17092. The POP gene and protein expression levels were detected using real-time PCR and Western blots, respectively. Oil red O staining was performed and the triglyceride levels in the L02 cells were also measured. Cell proliferation and apoptosis were detected using CCK-8 and flow cytometry, respectively. The expression of genes involved in lipid metabolism was detected using real-time PCR. The effects of POP inhibition on LC3B II were detected by Western blot.
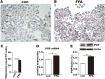
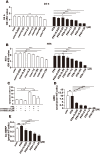
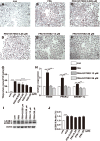
Results: Compared with the control, the POP mRNA levels increased by approximately 30%, and the POP protein levels increased by almost 60% in the steatotic L02 cells. After S17092 (0.026~130 μM) incubation for 24 or 48 h, cell proliferation was significantly decreased in the free fatty acid (FFA)-treated cells at 26-130 μM; however, S17092 did not affect the proliferation of L02 cells after 24 h of incubation with S17092 at 0.026-65 μM without FFA treatment. S17092 treatment (13 and 26 μM) also elicited no significant effect on apoptosis in normal L02 cells, but FFA treatment increased cell apoptosis, which was attenuated by S17092 incubation. S17092 treatment inhibited intracellular POP activity and decreased the AcSDKP level at the concentration of 0.026-26 μM. After treatment with FFA for 24 h, oil red O staining revealed significant lipid accumulation in the cells in the model group compared with the controls; however, lipid accumulation was suppressed after the administration of S17092 (13 and 26 μM). Accordingly, the triglyceride levels in the FFA-treated cells were approximately 5-fold greater than those of the controls and were decreased by approximately 25% and 45% after the administration of S17092 at 13 and 26 μM, respectively. The mRNA levels of FASN, PPAR-γ, and SREBP-1c were higher in the FFA-treated cells than in the normal controls, and all of these levels were significantly inhibited in the presence of S17092 at both 13 and 26 μM. S17092 treatment did not affect LC3B II in the FFA-treated cells compared with FFA treatment alone.
Conclusion: The expression of POP increases with hepatocyte steatosis, and POP inhibitors can significantly reduce intracellular lipid accumulation, which might be related to the inhibition of genes involved in lipid synthesis.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

