Bisphosphonate therapy for osteogenesis imperfecta
- PMID: 27760454
- PMCID: PMC6611487
- DOI: 10.1002/14651858.CD005088.pub4
Bisphosphonate therapy for osteogenesis imperfecta
Abstract
Background: Osteogenesis imperfecta is caused by a genetic defect resulting in an abnormal type I collagen bone matrix which typically results in multiple fractures with little or no trauma. Bisphosphonates are used in an attempt to increase bone mineral density and reduce these fractures in people with osteogenesis imperfecta. This is an update of a previously published Cochrane Review.
Objectives: To assess the effectiveness and safety of bisphosphonates in increasing bone mineral density, reducing fractures and improving clinical function in people with osteogenesis imperfecta.
Search methods: We searched the Cochrane Cystic Fibrosis and Genetic Disorders Group Inborn Errors of Metabolism Trials Register which comprises references identified from comprehensive electronic database searches, handsearches of journals and conference proceedings. We additionally searched PubMed and major conference proceedings.Date of the most recent search of the Cochrane Cystic Fibrosis and Genetic Disorders Group's Inborn Errors of Metabolism Register: 28 April 2016.
Selection criteria: Randomised and quasi-randomised controlled trials comparing bisphosphonates to placebo, no treatment, or comparator interventions in all types of osteogenesis imperfecta.
Data collection and analysis: Two authors independently extracted data and assessed the risk of bias of the included trials.
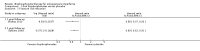
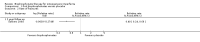
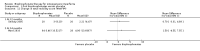
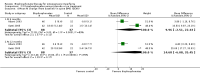
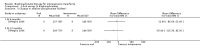
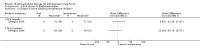
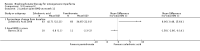
Main results: Fourteen trials (819 participants) were included. Overall, the trials were mainly at a low risk of bias, although selective reporting was an issue in several of the trials. Data for oral bisphosphonates versus placebo could not be aggregated; a statistically significant difference favouring oral bisphosphonates in fracture risk reduction and number of fractures was noted in two trials. No differences were reported in the remaining three trials which commented on fracture incidence. Five trials reported data for spine bone mineral density; all found statistically significant increased lumbar spine density z scores for at least one time point studied. For intravenous bisphosphonates versus placebo, aggregated data from two trials showed no statistically significant difference for the number of participants with at least one fracture, risk ratio 0.56 (95% confidence interval 0.30 to 1.06). In the remaining trial no statistically significant difference was noted in fracture incidence. For spine bone mineral density, no statistically significant difference was noted in the aggregated data from two trials, mean difference 9.96 (95% confidence interval -2.51 to 22.43). In the remaining trial a statistically significant difference in mean per cent change in spine bone mineral density z score favoured intravenous bisphosphonates at six and 12 months. Data describing growth, bone pain, and functional outcomes after oral or intravenous bisphosphonate therapy, or both, as compared to placebo were incomplete among all studies, but do not show consistent improvements in these outcomes. Two studies compared different doses of bisphosphonates. No differences were found between doses when bone mineral density, fractures, and height or length z score were assessed. One trial compared oral versus intravenous bisphosphonates and found no differences in primary outcomes. Two studies compared the intravenous bisphosphonates zoledronic acid and pamidronate. There were no significant differences in primary outcome. However, the studies were at odds as to the relative benefit of zoledronic acid over pamidronate for lumbosacral bone mineral density at 12 months.
Authors' conclusions: Bisphophonates are commonly prescribed to individuals with osteogenesis imperfecta. Current evidence, albeit limited, demonstrates oral or intravenous bisphosphonates increase bone mineral density in children and adults with this condition. These were not shown to be different in their ability to increase bone mineral density. It is unclear whether oral or intravenous bisphosphonate treatment consistently decreases fractures, though multiple studies report this independently and no studies report an increased fracture rate with treatment. The studies included here do not show bisphosphonates conclusively improve clinical status (reduce pain; improve growth and functional mobility) in people with osteogenesis imperfecta. Given their current widespread and expected continued use, the optimal method, duration of therapy and long-term safety of bisphosphonate therapy require further investigation. In addition, attention should be given to long-term fracture reduction and improvement in quality of life indicators.
Conflict of interest statement
All authors: none known.
Figures












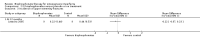



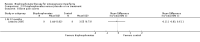

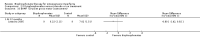





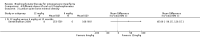

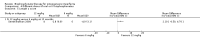



Update of
-
Bisphosphonate therapy for osteogenesis imperfecta.Cochrane Database Syst Rev. 2014 Jul 23;(7):CD005088. doi: 10.1002/14651858.CD005088.pub3. Cochrane Database Syst Rev. 2014. Update in: Cochrane Database Syst Rev. 2016 Oct 19;10:CD005088. doi: 10.1002/14651858.CD005088.pub4. PMID: 25054949 Updated.
References
References to studies included in this review
Adami 2003 {published and unpublished data}
-
- Adami S, Gatti D, Colapietro F, Fracassi E, Braga V, Rossini M, et al. Intravenous neridronate in adults with osteogenesis imperfecta. Journal of Bone and Mineral Research 2003;18(1):126‐30. - PubMed
-
- Gatti D, Viapiana O, Lippolis I, Braga V, Prizzi R, Rossini M, et al. Intravenous bisphosphonate therapy increases radial width in adults with osteogenesis imperfecta. Journal of Bone and Mineral Research 2005;20(8):1323‐6. - PubMed
Barros 2012 {published data only}
-
- Barros ER, Saraiva GL, Oliveira TP, Lazaretti‐Castro M. Safety and efficacy of a 1‐year treatment with zoledronic acid compared with pamidronate in children with osteogenesis imperfecta. Journal of Pediatric Endocrinology and Metabolism 2012;25(5‐6):485‐91. - PubMed
Bishop 2010 {published data only}
-
- Bishop N, Harrison R, Ahmed F, Shaw N, Eastell R, Campbell M, et al. A randomised controlled dose‐ranging study of risedronate in children with moderate and severe osteogenesis imperfecta. Journal of Bone and Mineral Research 2010;25(1):32‐40. - PubMed
Bishop 2013 {published data only}
-
- Bishop N, Adami S, Ahmed SF, Antón J, Arundel P, Burren CP, et al. Risedronate in children with osteogenesis imperfecta:a randomised, double‐blind, placebo‐controlled trial. Lancet 2013;382(9902):1424‐32. - PubMed
Chevrel 2006 {published data only}
-
- Chevrel G, Schott AM, Fontanges E, Charrin JE, Lina‐Granade G, Duboeuf F, et al. Effects of oral alendronate on BMD in adult patients with osteogenesis imperfecta: a 3‐year randomized placebo‐controlled trial. Journal of Bone and Mineral Research 2006;21(2):300‐6. - PubMed
DiMeglio 2006 {published data only}
-
- DiMeglio LA, Peacock M. Two‐year clinical trial of oral alendronate versus intravenous pamidronate in children with osteogenesis imperfecta. Journal of Bone and Mineral Research 2006;21(1):132‐40. - PubMed
-
- Dimeglio LA, Ford L, McClintock C, Peacock M. A comparison of oral and intravenous bisphosphonate therapy for children with osteogenesis imperfecta. Journal of Pediatric Endocrinology and Metabolism 2005;18(1):43‐53. - PubMed
Gatti 2005 {published and unpublished data}
-
- Gatti D, Antoniazzi F, Prizzi R, Braga V, Rossini M, Tato L, et al. Intravenous neridronate in children with osteogenesis imperfecta: A randomized controlled study. Journal of Bone and Mineral Research 2005;20(5):758‐63. - PubMed
Letocha 2005 {published data only}
-
- Letocha AD, Cintas HL, Troendle JF, Reynolds JC, Cann CE, Chernoff EJ, et al. Controlled trial of pamidronate in children with types III and IV osteogenesis imperfecta confirms vertebral gains but not short‐term functional improvement. Journal of Bone and Mineral Research 2005;20(6):977‐86. - PubMed
Rauch 2009 {published data only}
-
- Rauch F, Munns CF, Land C, Cheung M, Glorieux FH. Risedronate in the treatment of mild pediatric osteogenesis imperfecta: a randomized placebo‐controlled study. Journal of Bone and Mineral Research 2009;24(7):1282–9. - PubMed
Sakkers 2004 {published data only}
-
- Sakkers R, Kok D, Engelbert R, Dongen A, Jansen M, Pruijs H, et al. Skeletal effects and functional outcome with olpadronate in children with osteogenesis imperfecta: a 2‐year randomised placebo‐controlled study. Lancet 2004;363(9419):1427‐31. [] - PubMed
Seikaly 2005 {published data only}
-
- Seikaly MG, Kopanati S, Salhab N, Waber P, Patterson D, Browne R, et al. Impact of alendronate on quality of life in children with osteogenesis imperfecta. Journal of Pediatric Orthopaedics 2005;25(6):786‐91. - PubMed
Senthilnathan 2008 {published data only}
-
- Senthilnathan S, Walker E, Bishop NJ. Two doses of pamidronate in infants with osteogenesis imperfecta. Archives of Disease in Childhood 2008;93(5):398‐400. - PubMed
Ward 2010 {published data only}
-
- Glorieux FH, Rauch F, Ward LM, Smith P, Verbruggen N, Heydon N, et al. Alendronate in the treatment of pediatric osteogenesis imperfecta [abstract]. Journal of Bone and Mineral Research 2004;20 Suppl:S12. []
-
- Ward LM, Rauch F, Whyte MP, D'Astous J, Gates PE, Grogan D, et al. Alendronate for the treatment of pediatric osteogenesis imperfecta: a randomized placebo‐controlled study. Journal of Clinical Endocrinology and Metabolism 2011;96(2):355‐64. - PubMed
Zoledronic Acid 2008 {unpublished data only}
-
- Food, Drug Administration (FDA). Center for Drug Evaluation and Research. Statistical review and evaluation ‐ clinical studies: Zometa (zoledronic acid) intravenous injection (i.v.) 4 mg lyophilized powder. Treatment of children with osteogenesis imperfecta (OI). http://www.fda.gov/downloads/drugs/developmentapprovalprocess/developmen... (accessed 07 April 2014).
-
- NCT00063479. Bisphosphonate treatment of osteogenesis imperfecta. http://clinicaltrials.gov/show/NCT00063479 (accessed 08 March 2013).
References to studies excluded from this review
Antoniazzi 1996 {published data only}
-
- Antoniazzi F, Bertoldo F, Mottes M, Valli M, Sirpresi S, Zamboni G, et al. Growth hormone treatment in osteogenesis imperfecta with quantitative defect of type I collagen synthesis. Journal of Pediatrics 1996;129(3):432‐9. - PubMed
Antoniazzi 2006 {published data only}
-
- Antoniazzi F, Zamboni G, Lauriola S, Donadi L, Adami S, Tato L. Early bisphosphonate treatment in infants with severe osteogenesis imperfecta. Journal of Pediatrics 2006;149(2):174‐9. - PubMed
Antoniazzi 2010 {published data only}
-
- Antoniazzi F, Monti E, Venturi G, Franceschi R, Doro F, Gatti D, et al. GH in combination with bisphosphonate treatment in osteogenesis imperfecta. European Journal of Endocrinology 2010;163(3):479‐87. - PubMed
DiMeglio 2004 {published data only}
-
- DiMeglio LA, Ford L, McClintock C, Peacock M. Intravenous pamidronate treatment of children under 36 months of age with osteogenesis imperfecta. Bone 2004;35(5):1038‐45. - PubMed
Gerber 1998 {published data only}
-
- Gerber LH, Binder H, Berry R, Siegel KL, Kim H, Weintrob J, et al. Effect of withdrawal of bracing in matched pairs of children with osteogenesis imperfecta. Archives of Physical Medicine and Rehabilitation 1998;79(1):46‐51. - PubMed
Granda 1977 {published data only}
-
- Granda JL, Falva KA, Bullough PG. Phyrophosphate levels and magnesium oxide therapy in osteogenesis imperfecta. Clinical Orthopaedics and Related Research 1977;Jul‐Aug(216):228‐31. - PubMed
Orwoll 2014 {unpublished data only}
-
- NCT00131469. Study of Teriparatide (FORTEO) to Treat Adults With Osteogenesis Imperfecta (OI). http://clinicaltrials.gov/show/NCT00131469 (accessed 08 March 2013). []
-
- Nagamani S, Shapiro J, Veith S, Wang Y, Lapidus J, Reeder JL, et al. Teriparatide, the first anabolic agent for treatment of osteogenesis imperfecta improves bone mineral density at the hip and spine: a randomized, blinded, placebo‐controlled trial [abstract]. Proceedings of the 63rd Annual Meeting of the American Society of Human Genetics; 2013 Oct 22‐26; Boston. Boston: American Society of Human Genetics, 2013:27.
Ward 2005 {published data only}
-
- Ward LM, Denker AE, Porras A, Shugarts S, Kline W, Travers R, et al. Single‐dose pharmacokinetics and tolerability of alendronate 35‐ and 70‐milligram tablets in children and adolescents with osteogenesis imperfecta type I. Journal of Clinical Endocrinology and Metabolism 2005;90(7):4051‐6. - PubMed
Additional references
Alanay 2010
Barnes 2006
Becker 2011
Black 1996
-
- Black DM, Cummings SR, Karpf DB, Cauley JA, Thompson DE, Nevitt MC, et al. Randomized trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Lancet 1996;348(9041):1535‐41. - PubMed
Byers 1991
Byers 1992
-
- Byers PH, Steiner RD. Osteogenesis imperfecta. Annual Review of Medicine 1992;43:269‐82. - PubMed
Byers 2000
-
- Byers PH. Osteogenesis imperfecta: perspectives and opportunities. Current Opinion in Pediatrics 2000;12(6):603‐9. - PubMed
Byers 2013
-
- Byers 2013 [pers comm]. OI [personal communication]. Email to: D Basel 2013.
Cabral 2007
Camacho 2001
-
- Camacho NP, Raggio CL, Doty SB, Root L, Zraick V, Ilg WA, et al. A controlled study of the effects of alendronate in a growing mouse model of osteogenesis imperfecta. Calcified Tissue International 2001;69(2):94‐101. - PubMed
Castillo 2009
-
- Castillo H, Samson‐Fang L, American Academy for Cerebral Palsy and Developmental Medicine Treatment Outcomes Committee Review Panel. Effects of bisphosphonates in children with osteogenesis imperfecta: an AACPDM systematic review. Developmental Medicine and Child Neurology 2009;51(1):17‐29. - PubMed
Cho 2012
Christiansen 2010
-
- Christiansen HE, Schwarze U, Pyott SM, AlSwaid A, Al Balwi M, Alrasheed S, et al. Homozygosity for a missense mutation in SERPINH1, which encodes the collagen chaperone protein HSP47, results in severe recessive osteogenesis imperfecta. American Journal of Human Genetics 2010;86(3):389‐98. - PMC - PubMed
Cole 2002
-
- Cole WG. Advances in Osteogenesis Imperfecta. Clinical Orthopaedics and Related Research 2002;401:6‐16. - PubMed
Connor 1985
-
- Connor JM, Connor RA, Sweet EM, Gibson AA, Patrick WJ, McNay MB, et al. Lethal neonatal chondrodysplasias in the West of Scotland 1970‐1983 with a description of a thanatophoric, dysplasialike, autosomal recessive disorder, Glasgow variant. American Journal of Medical Genetics 1985;22(2):243‐53. - PubMed
Elbourne 2002
-
- Elbourne DR, Altman DG, Higgins JP, Curtin F, Worthington HV, Vail A. Meta‐analyses involving cross‐over trials: methodological issues. International Journal of Epidemiology 2002;31(1):140‐9. - PubMed
Erviti 2013
-
- Erviti J, Alonso A, Oliva B, Gorricho J, López A, Timoner J, et al. Oral bisphosphonates are associated with increased risk of subtrochanteric and diaphyseal fractures in elderly women: a nested case–control study. http://bmjopen.bmj.com/content/3/1/e002091 (accessed 06 February 2013). - PMC - PubMed
FDA 2011
-
- Food, Drug Administration (FDA). Background document for meeting of Advisory Committee for Reproductive Health Drugs and Drug Safety and Risk Management Advisory Committee. http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMateria... (accessed 07 March 2013).
Fisher 1999
-
- Fisher JE, Rogers MJ, Halasy JM, Luckman SP, Hughes DE, Masarachia PJ, et al. Alendronate mechanism of action: geranylgeraniol, an intermediate in the mevalonate pathway, prevents inhibition of osteoclast formation, bone resorption, and kinase activation in vitro [abstract]. Proceedings of the National Academy of Sciences of the United States of America 1999;96:133‐8. - PMC - PubMed
Gatti 2013
-
- Gatti D, Rossini M, Viapiana O, Povino MR, Liuzza S, Fracassi E, et al. Teriparatide treatment in adult patients with osteogenesis imperfecta type I. Calcified Tissue International 2013;93(5):448‐52. - PubMed
Glorieux 2000
-
- Glorieux FH, Rauch F, Plotkin H, Ward L, Travers R, Roughley P, et al. Type V osteogenesis imperfecta: a new form of brittle bone disease. Journal of Bone and Mineral Research 2000;15(9):1650‐8. - PubMed
Glorieux 2004
-
- Glorieux FH, Rauch F, Ward LM, Smith P, Verbruggen N, Heyden N, et al. Alendronate in the treatment of pediatric osteogenesis imperfecta [abstract]. Journal of Bone and Mineral Research 2004;20(Suppl):1043.
Higgins 2003
Higgins 2011a
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration 2011. Available from www.cochrane‐handbook.org.
Higgins 2011b
-
- Higgins JPT, Altman DG (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Jüni 2001
Kuurila 2002
-
- Kuurila K, Kaitila I, Johansson R, Grenman R. Hearing loss in Finnish adults with osteogenesis imperfecta: a nationwide survey. The Annals of Otology, Rhinology, and Laryngology 2002;111(10):939‐46. - PubMed
Lapunzina 2010
Marini 2003
-
- Marini JC. Do bisphosphonates make children's bones better or brittle?. New England Journal of Medicine 2003;349(5):423‐6. - PubMed
Martinez‐Glez 2012
OIF 2008
-
- Osteogenesis Imperfecta Foundation. Facts about Osteogenesis Imperfecta. www.oif.org (accessed August 2008).
Orioli 1995
-
- Orioli IM, Castilla EE, Scarano G, Mastroiacovo P. Effect of paternal age in achondroplasia, thanatophoric dysplasia, and osteogenesis imperfecta. American Journal of Medical Genetics 1995;59(2):209‐17. - PubMed
Pyott 2013
Reid 2002
-
- Reid IR, Brown JP, Burckhardt P, Horowitz Z, Richardson P, Trechsel U, et al. Intravenous zoledronic acid in postmenopausal women with low bone mineral density. New England Journal of Medicine 2002;346(9):653‐61. - PubMed
Semler 2012
Sillence 1979
Sillence 2012
-
- Sillence D. Osteogenesis Imperfecta. International committee for constitutional disorders of the skeleton. Sydney, 2012.
Steiner 2013
-
- Steiner RD, Adsit J, Basel D. Gene Reviews; COL1A1/2‐Related Osteogenesis Imperfecta. http://www.ncbi.nlm.nih.gov/books/NBK1295/ (Initial Posting: January 28, 2005; Last Update: February 14, 2013).
Thornton 2006
Van Dijk 2009
Whyte 2003
-
- Whyte MP, Wenkert D, Clements KL, McAlister WH, Mumm S. Bisphosphonate‐induced osteopetrosis. New England Journal of Medicine 2003;349(5):457‐63. - PubMed
Woo 2006
-
- Woo SB, Hellstein JW, Kalmar JR. Narrative [corrected] review: bisphosphonates and osteonecrosis of the jaws. Annals of Internal Medicine 2006;144(10):753‐61. - PubMed
References to other published versions of this review
Dwan 2014
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

