A small molecule screening to detect potential therapeutic targets in human podocytes
- PMID: 27760769
- PMCID: PMC5504421
- DOI: 10.1152/ajprenal.00386.2016
A small molecule screening to detect potential therapeutic targets in human podocytes
Abstract
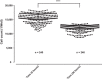
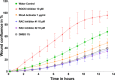
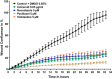
A small molecule screening to detect potential therapeutic targets in human podocytes. Am J Physiol Renal Physiol 312: F157-F171, 2017. First published October 19, 2016; doi:10.1152/ajprenal.00386.2016. Steroid-resistant nephrotic syndrome (SRNS) inevitably progresses to end-stage kidney disease, requiring dialysis or transplantation for survival. However, treatment modalities and drug discovery remain limited. Mutations in over 30 genes have been discovered as monogenic causes of SRNS. Most of these genes are predominantly expressed in the glomerular epithelial cell, the podocyte, placing it at the center of the pathogenesis of SRNS. Podocyte migration rate (PMR) represents a relevant intermediate phenotype of disease in monogenic causes of SRNS. We therefore adapted PMR in a high-throughput manner to screen small molecules as potential therapeutic targets for SRNS. We performed a high-throughput drug screening of a National Institutes of Health Clinical Collection (NCC) library (n = 725 compounds) measuring PMR by videomicroscopy. We used the Woundmaker to perform individual 96-well scratch wounds and screened compounds using a quantitative kinetic live cell imaging migration assay using IncuCyte ZOOM technology. Using a normal distribution for the average PMR in wild-type podocytes with a vehicle control (DMSO), we applied a 90% confidence interval to define "distinct" compounds (5% faster/slower PMR) and found that 12 of 725 compounds (at 10 μM) reduced PMR. Clusters of drugs that alter PMR included actin/tubulin modulators such as the azole class of antifungals and antineoplastic vinca-alkaloids. We hereby identify compounds that alter PMR. The PMR assay provides a new avenue to test therapeutics for nephrotic syndrome. Positive results may reveal novel pathways in the study of glomerular diseases such as SRNS.
Keywords: podocyte; small molecule screen; steroid-resistant nephrotic syndrome.
Copyright © 2017 the American Physiological Society.
Figures




Comment in
-
Stop that podocyte!Am J Physiol Renal Physiol. 2017 Feb 1;312(2):F373-F374. doi: 10.1152/ajprenal.00499.2016. Epub 2016 Oct 19. Am J Physiol Renal Physiol. 2017. PMID: 27760773 Free PMC article. No abstract available.
References
-
- Alvarez J, Montero M, Garcia-Sancho J. High affinity inhibition of Ca(2+)-dependent K+ channels by cytochrome P-450 inhibitors. J Biol Chem 267: 11789–11793, 1992. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

